Can Genetic Testing Help Shed Light on the Autism Epidemic?

A little boy standing by a window in contemplation. (© altanaka/Fotolia)
Autism cases are still on the rise, and scientists don't know why. In April, the Centers for Disease Control (CDC) reported that rates of autism had increased once again, now at an estimated 1 in 59 children up from 1 in 68 just two years ago. Rates have been climbing steadily since 2007 when the CDC initially estimated that 1 in 150 children were on the autism spectrum.
Some clinicians are concerned that the creeping expansion of autism is causing the diagnosis to lose its meaning.
The standard explanation for this increase has been the expansion of the definition of autism to include milder forms like Asperger's, as well as a heightened awareness of the condition that has improved screening efforts. For example, the most recent jump is attributed to children in minority communities being diagnosed who might have previously gone under the radar. In addition, more federally funded resources are available to children with autism than other types of developmental disorders, which may prompt families or physicians to push harder for a diagnosis.
Some clinicians are concerned that the creeping expansion of autism is causing the diagnosis to lose its meaning. William Graf, a pediatric neurologist at Connecticut Children's Medical Center, says that when a nurse tells him that a new patient has a history of autism, the term is no longer a useful description. "Even though I know this topic extremely well, I cannot picture the child anymore," he says. "Use the words mild, moderate, or severe. Just give me a couple more clues, because when you say autism today, I have no idea what people are talking about anymore."
Genetic testing has emerged as one potential way to remedy the overly broad label by narrowing down a heterogeneous diagnosis to a specific genetic disorder. According to Suma Shankar, a medical geneticist at the University of California, Davis, up to 60 percent of autism cases could be attributed to underlying genetic causes. Common examples include Fragile X Syndrome or Rett Syndrome—neurodevelopmental disorders that are caused by mutations in individual genes and are behaviorally classified as autism.
With more than 500 different mutations associated with autism, very few additional diagnoses provide meaningful information.
Having a genetic diagnosis in addition to an autism diagnosis can help families in several ways, says Shankar. Knowing the genetic origin can alert families to other potential health problems that are linked to the mutation, such as heart defects or problems with the immune system. It may also help clinicians provide more targeted behavioral therapies and could one day lead to the development of drug treatments for underlying neurochemical abnormalities. "It will pave the way to begin to tease out treatments," Shankar says.
When a doctor diagnoses a child as having a specific genetic condition, the label of autism is still kept because it is more well-known and gives the child access to more state-funded resources. Children can thus be diagnosed with multiple conditions: autism spectrum disorder and their specific gene mutation. However, with more than 500 different mutations associated with autism, very few additional diagnoses provide meaningful information. What's more, the presence or absence of a mutation doesn't necessarily indicate whether the child is on the mild or severe end of the autism spectrum.
Because of this, Graf doubts that genetic classifications are really that useful. He tells the story of a boy with epilepsy and severe intellectual disabilities who was diagnosed with autism as a young child. Years later, Graf ordered genetic testing for the boy and discovered that he had a mutation in the gene SYNGAP1. However, this knowledge didn't change the boy's autism status. "That diagnosis [SYNGAP1] turns out to be very specific for him, but it will never be a household name. Biologically it's good to know, and now it's all over his chart. But on a societal level he still needs this catch-all label [of autism]," Graf says.
"It gives some information, but to what degree does that change treatment or prognosis?"
Jennifer Singh, a sociologist at Georgia Tech who wrote the book Multiple Autisms: Spectrums of Advocacy and Genomic Science, agrees. "I don't know that the knowledge gained from just having a gene that's linked to autism," is that beneficial, she says. "It gives some information, but to what degree does that change treatment or prognosis? Because at the end of the day you have to address the issues that are at hand, whatever they might be."
As more children are diagnosed with autism, knowledge of the underlying genetic mutation causing the condition could help families better understand the diagnosis and anticipate their child's developmental trajectory. However, for the vast majority, an additional label provides little clarity or consolation.
Instead of spending money on genetic screens, Singh thinks the resources would be better used on additional services for people who don't have access to behavioral, speech, or occupational therapy. "Things that are really going to matter for this child in their future," she says.
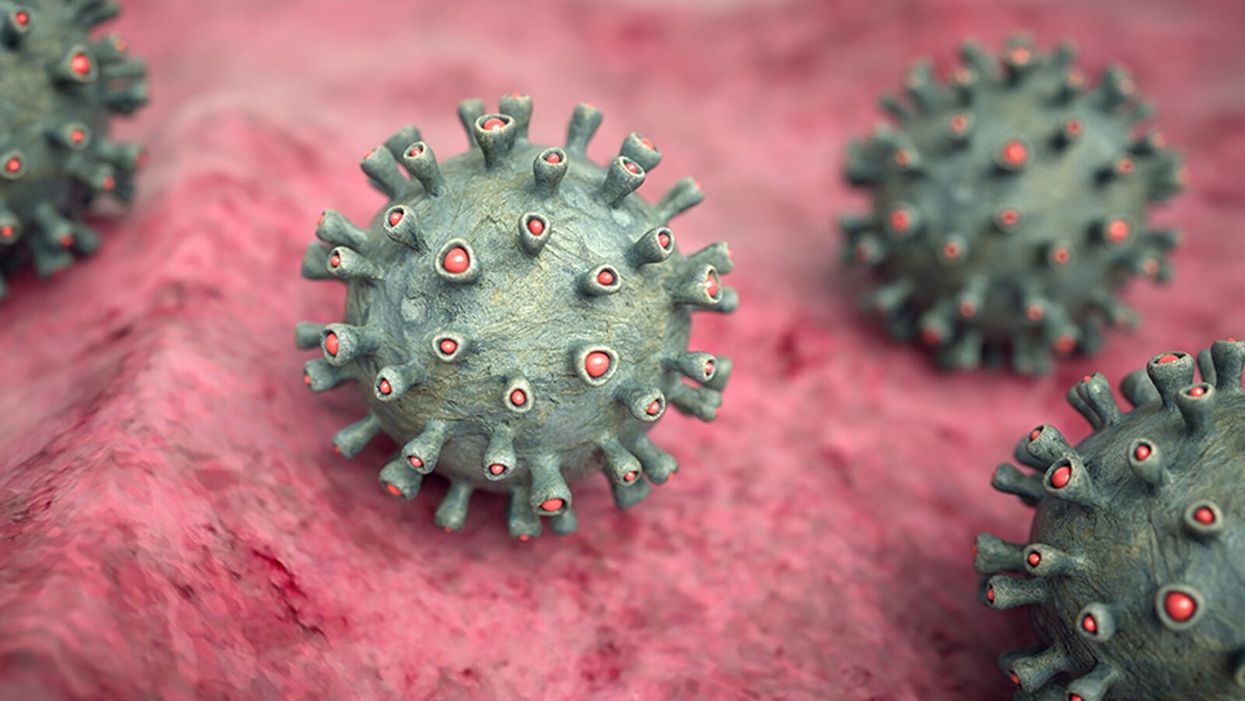
Would a Broad-Spectrum Antiviral Drug Stop the Pandemic?
An illustration of the novel coronavirus infecting human tissue.
The refocusing of medical research to COVID-19 is unprecedented in human history. Seven months ago, we barely were aware that the virus existed, and now a torrent of new information greets us each day online.
There are many unanswered questions about COVID-19, but perhaps the most fascinating is whether we even need to directly go after the virus itself.
Clinicaltrials.gov, the most commonly used registry for worldwide medical research, listed 1358 clinical trials on the disease, including using scores of different potential drugs and multiple combinations, when I first wrote this sentence. The following day that number of trials had increased to 1409. Laboratory work to prepare for trials presents an even broader and untabulated scope of activity.
Most trials will fail or not be as good as what has been discovered in the interim, but the hope is that a handful of them will yield vaccines for prevention and treatments to attenuate and ultimately cure the deadly infection.
The first impulse is to grab whatever drugs are on the shelf and see if any work against the new foe. We know their safety profiles and they have passed some regulatory hurdles. Remdesivir is the first to register some success against SARS-CoV-2, the virus behind the disease. The FDA has granted it expedited-use status, pending presentation of data that may lead to full approval of the drug.
Most observers see it as a treatment that might help, but not one that by itself is likely to break the back of the pandemic. Part of that is because it is delivered though IV infusion, which requires hospitalization, and as with most antiviral drugs, appears to be most beneficial when started early in disease. "The most effective products are going to be that ones that are developed by actually understanding more about this coronavirus," says Margaret "Peggy" Hamburg, who once led the New York City public health department and later the U.S. Food and Drug Administration.
Combination therapy that uses different drugs to hit a virus at different places in its life cycle have proven to work best in treating HIV and hepatitis C, and likely will be needed with this virus as well. Most viruses are simply too facile at evolving resistance to a single drug, and so require multiple hits to keep them down.
Laboratory work suggests that other drugs, both off-the-shelf and in development, particularly those to treat HIV and hepatitis, might also be of some benefit against SARS-CoV-2. But the number of possible drug combinations is mind-bogglingly large and the capacity to test them all right now is limited.
Broad-Spectrum Antivirals
Viruses are simple quasi-life forms. Effective treatments are more likely to be specific to a given virus, or at best its close relatives. That is unlike bacteria, where broad-spectrum antibiotics often can be used against common elements like the bacterial cell wall, or can disrupt quorum sensing signals that bacteria use to function as biofilms.
More than a decade ago, virologist Benhur Lee's lab at UCLA (now at Mt. Sinai in New York City) stumbled upon a broad-spectrum antiviral approach that seemed to work against all enveloped viruses they tested. The list ranged from the common flu to HIV to Ebola.
Other researchers grabbed this lead to develop a compound that worked quite well in cell cultures, but when they tried it in animals, a frustrating snag emerged; the compound needed to be activated by light. As the greatest medical need is to counter viruses deep inside the body, the research was put on the shelf. So Lee was surprised to learn recently that a company has inquired about rights to develop the compound not as a treatment but as a possible disinfectant. The tale illustrates both the unanticipated difficulties of drug development and that one never knows how knowledge ultimately might be put to use.
Remdesivir is a failed drug for Ebola that has found new life with SARS-CoV-2. It targets polymerase, an enzyme that the virus produces to use host cell machinery to replicate itself, and since the genetic sequence of polymerase is very similar among all of the different coronaviruses, scientists hope that the drug might be useful against known members of the family and others that might emerge in the future.
But nature isn't always that simple. Viral RNA is not a two-dimensional assemblage of genes in a flat line on a table; rather it is a three-dimensional matrix of twists and turns where a single atom change within the polymerase gene or another gene close by might change the orientation of the RNA or a molecular arm within it and block a drug from accessing the targeted binding site on the virus. One drug might need to bind to a large flat surface, while another might be able to slip a dagger-like molecular arm through a space in the matrix to reach its binding target.
That is why a broad-spectrum antiviral is so hard to develop, and why researchers continue to work on a wide variety of compounds that target polymerase as a binding site.
Additionally, it has taken us decades to begin to recognize the unintended consequences of broad-spectrum rather than narrowly targeted antibiotics on the gut microbiome and our overall health. Will a similar issue potentially arise in using a broad-spectrum antiviral?
"Off-target side effects are always of concern with drugs, and antivirals are no exception," says Yale University microbiologist Ben Chen. He believes that "most" bacteriophages, the viruses that infect bacteria and likely help to maintain stability in the gut microbial ecosystem, will shrug off such a drug. However, a few families of phages share polymerases that are similar to those found in coronaviruses. While the immediate need for treatment is great, we will have to keep a sharp eye out for unanticipated activity in the body's ecosystem from new drugs.
Is an Antiviral Needed?
There are many unanswered questions about COVID-19, but perhaps the most fascinating is whether we even need to directly go after the virus itself. Mounting evidence indicates that up to half the people who contract the infection don't seem to experience significant symptoms and their immune system seems to clear the virus.
The most severe cases of COVID-19 appear to result from an overactive immune response that damages surrounding tissue. Perhaps downregulating that response will be sufficient to reduce the disease burden. Several studies are underway using approved antibodies that modulate an overly active immune response.
One of the most surprising findings to date involves the monoclonal antibody leronlimab. It was originally developed to treat HIV infection and works modestly well there, but other drugs are better and its future likely will be mainly to treat patients who have developed resistance to those other drugs.
The response has been amazingly different in patients in the U.S. with COVID-19 who were given emergency access to leronlimab – two injections a week apart, though the company believes that four might be better. The immune response and inflammatory cytokines declined significantly, T cell counts were maintained, and surprisingly the amount of virus in the blood declined too. Data from the first ten patients is available in a preprint while the paper undergoes peer review for publication. Data from an additional fifty patients will be added.
"We got lucky and hit the bulls' eye from a mile away," says Jay Lalezari, the chief science officer of Cytodyn, the company behind leronlimab. Dr. Jay, as he is widely known in San Francisco, built an adoring fan base running many of the early-phase drug studies for treating HIV. While touting leronlimab, Lalezari suspects it might best be used as part of a combination therapy.
The small, under-capitalized firm is struggling for attention in the vast pool of therapies proposed to treat COVID-19. It faces the added challenge of gaining acceptance because it is based on a different approach and mechanism of action, which involves a signaling molecule important to immune cell migration, than what most researchers and the FDA anticipate as being relevant to counter SARS-CoV-2.
Common Issues
All of the therapeutics under development will face some common sets of issues. One is the pressure to have results yesterday, because people are dying. The rush to disseminate information "make me worry that certain things will become entrenched as truth, even in the scientific community, without the actual scientific documentation that ordinarily scientists would demand," says Hamburg.
"It is becoming increasingly clear that the biggest problem for drug and vaccine makers is not which therapeutics or vaccine platform to pursue."
Lack of standardization in assays and laboratory operations makes it difficult to compare results between labs studying SARS-CoV-2. In the long run, this will slow down the iterative process of research that builds upon what has gone before. And the shut down of supply chains, from chemicals to cell lines to animals to air shipment, has the potential to further hobble research.
Almost all researchers consult with the FDA in putting together their clinical trials. But the agency is overwhelmed with the surge of activity in the field, and is even less capable of handling novel approaches that fall outside of its standard guidance.
"It is becoming increasingly clear that the biggest problem for drug and vaccine makers is not which therapeutics or vaccine platform to pursue. It is that conventional clinical development paths are far too lengthy and cumbersome to address the current public health threat," John Hodgson wrote in Nature Biotechnology.
Another complicating factor with this virus is the broad range of organ and tissue types it can infect. That has implications for potential therapies, which often vary in their ability to enter different tissues. At a minimum, it complicates the drug development process.
Remdesivir has become the de facto standard of care. Ideally, clinical trials are conducted using the existing standard of care rather than a placebo as the control group. But shortages of the drug make that difficult and further inhibit learning what is the best treatment regimen for regular clinical care.
"Understandably, we all really want to respond to COVID-19 in a much, much more accelerated fashion," says Hamburg. But ultimately that depends upon "the reality of understanding the nature of the disease. And that is going to take a bit more time than we might like or wish."
[This article was originally published on June 8th, 2020 as part of a standalone magazine called GOOD10: The Pandemic Issue. Produced as a partnership among LeapsMag, The Aspen Institute, and GOOD, the magazine is available for free online.]
Anne, Stan, and grandson Louie during vacation in Mexico, 2019. INSET: Anne post-op in 2017.
[Editor's Note: On June 6, 2017, Anne Shabason, an artist, hospice educator, and mother of two from Bolton, Ontario, a small town about 30 miles outside of Toronto, underwent Deep Brain Stimulation (DBS) to treat her Parkinson's disease. The FDA approved DBS for Parkinson's disease in 2002. Although it's shown to be safe and effective, agreeing to invasive brain surgery is no easy decision, even when you have your family and one of North America's premier neurosurgeons at your side.
Here, with support from Stan, her husband of the past 40 years, Anne talks about her life before Parkinson's, what the disease took away, and what she got back because of DBS. As told to writer Heather R. Johnson.]
I was an artist.
I worked in mixed media, Papier-mâché, and collage, inspired by dreams, birds, mystery. I had gallery shows and participated in studio tours.
Educated in thanatology, I worked in hospice care as a volunteer and education director for Hospice Caledon, an organization that supports people facing life-limiting illness and grief.
I trained volunteers who helped people through their transition.
Parkinson's disease changed all that.
My hands and my head were not coordinating, so it was impossible to do my art.
It started as a twitch in my leg. During a hospice workshop, my right leg started vibrating in a way I hadn't experienced before. I told a friend, "This can't be good."
Over the next year, my right foot vibrated more and more. I could not sleep well. In my dreams people lurked in corners, in dark places, and behind castle doors. I knew they were there and couldn't avoid the ambush. I shrieked and woke everyone in the house.
An anxiety attack—something I had also never experienced before—came next.
During a class I was teaching, my mouth got so dry, I couldn't speak. I stood in front of the class for three or four minutes, unable to continue. I pushed through and finished the class. That's when I realized this was more than jiggling legs.
That's when I went to see a doctor.
A Diagnosis
My first doctor, when I suggested it might be Parkinson's, didn't believe me. She sent me to a neurologist who told me I had to meditate more and calm myself.
A friend from hospice told me to phone the Toronto Western Hospital Movement Disorders Clinic. In January 2010, I was diagnosed with Parkinson's disease.
The doctor, a fellow, got all my stats and asked a lot of questions. He was so excited he knew what it was, he exclaimed, "You've got Parkinson's!" like it was the best thing ever. I must say, that wasn't the best news, but at least I finally had a diagnosis.
I could choose whether to take medication or not. The doctor said, "If Parkinson's is compromising your lifestyle, you should consider taking levodopa."
"Well I can't run my classes, I can't do my art, so it's compromising me," I said. And my health was going downhill. The shaking—my whole body moved—sleeping was horrible. Two to four hours max a night was usual. I had terrible anxiety and panic attacks and had to quit work.
So I started taking levodopa. It's taken in a four-hour cycle, but the medication didn't last the full time. I developed dyskenisia, a side effect of the medication that made me experience uncontrolled, involuntary movements. I was edgy, irritable, and focused on my watch like a drug addict. I'd lie on the couch, feel crummy and tired, and wait.
The medication cycle restricted where I could go. Fearing the "off" period, I avoided interaction with lifelong friends, which increased my feeling of social isolation. They would come over and cook with me and read to me sometimes, and that was fine, as long as it was during an "on" period.
There was incontinence, constipation, and fatigue.
I lost fine motor skills, like writing. And painting. My hands and my head were not coordinating, so it was impossible to do my art.
It was a terrible time.
The worst symptoms—what pushed me to consider DBS—were the symptoms no one could see. The anxiety and depression were so bad, the sleeplessness, not eating.
I projected a lot of my discomforts onto Stan. I reacted so badly to him. I actually separated from him briefly on two separate occasions and lived in a separate space—a self-imposed isolation. There wasn't anything he could do to help me really except sit back and watch.
I tried alternative therapies—a naturopath, an osteopath, a reflexologist and a Chinese medicine practitioner—but nothing seemed to help.
I felt like I was dying. Certain parts of my life were being taken away from me. I was a perfectionist, and I felt imperfect. It was a horrible feeling, to not be in control of myself.
The DBS Decision
I was familiar with DBS, a procedure that involves a neurosurgeon drilling small holes into your skull and implanting electrical leads deep in your brain to modify neural activity, reducing involuntary movements.
But I was convinced I'd never do it. I was brought up in a family that believed 'doctors make you sick and hospitals kill you.'
I worried the room wouldn't be sterile. Someone's cutting into your brain, you don't know what's going to happen. They're putting things in your body. I didn't want to risk possible infection.
And my doctor said he couldn't promise he would actually do the operation. It might be a fellow, but he'd be in the background in case anything went wrong. I wasn't comfortable with that arrangement.
When filmmakers Taryn Southern and Elena Gaby decided to make a documentary about people whose lives were changed by cutting-edge brain implants--and I agreed to participate—my doctor said he would for sure do the operation. They couldn't risk anything happening on the operating table on camera, so most of my fears went away.
My family supported the decision. My mother had trigeminal neuralgia, which is a very painful facial condition. She also had a stroke and what we now believe to be Parkinson's. My father, a retired dentist, managed her care and didn't give her the opportunity to see a specialist.
I felt them running the knife across my scalp, and drilling two holes in my head, but only as pressure, not pain.
When we were talking about DBS, my son, Joseph, said, "How can you not do this, for the sake of your family? Because if you don't, you'll end up like Grandma, who, for the last few years of her life, just lay on a couch because she didn't get any kind of outside help. If you even have a chance to improve your life or give yourself five extra years, why wouldn't you do that, for our sake? Are we not worth that?"
That talk really affected me, and I realized I had to try. Even though it was difficult, I had to be brave for my family.
Surgery, Recovery, and Tweaking
You have to be awake for part of the procedure—I was awake enough that my subconscious could hear, because they had to know how far to insert the electrodes. DBS targets the troublemaking areas of the brain. There's a one millimeter difference between success and failure.
I felt them running the knife across my scalp, and drilling two holes in my head, but only as pressure, not pain.
Once they were inside, they asked me to move parts of my body to see whether the right neurons were activated.
They put me to sleep to put a battery-powered neurostimulator in my chest. A wire that runs behind my ear and down my neck connects the electrodes in my brain to the battery pack. The neurostimulator creates electric pulses 24 hours a day.
I was moving around almost immediately after surgery. Recovery from the stitches took a few weeks, but everything else took a lot longer.
I couldn't read. My motor skills were still impaired, and my brain and my hands weren't yet linked up. I needed the device to be programmed and tweaked. Until that happened, I needed help.
The depression and anxiety, though, went away almost immediately. From that perspective, it was like I never had Parkinson's. I was so happy.
When they calibrated the electrodes, they adjusted how much electrical current goes to any one of four contact points on the left and right sides of the brain. If they increased it too much, a leg would start shaking, a foot would start cramping, or my tongue would feel thicker. It took a while to get it calibrated correctly to control the symptoms.
First it was five sessions in five weeks, then once a month, then every three months. Now I visit every six months. As the disease progresses, they have the ability to keep making adjustments. (DBS controls the symptoms, but it doesn't cure the disease.)
Once they got the calibration right, my motor skills improved. I could walk without shuffling. My muscles weren't stiff and aching, and the dyskinesia disappeared. But if I turn off the device, my symptoms return almost immediately.
Some days I have more fatigue than others, and sometimes my brain doesn't click. And my voice got softer – that's a common side effect of this operation. But I'm doing so much better than before.
I have a quality of life I didn't have before. Before COVID-19 hit, Stan and I traveled, went to concerts, movies, galleries, and spent time with our growing family.
I cut back the levodopa from seven-and-a-half pills a day to two-and-a-half. I often forget to take my medication until I realize I'm feeling tired or anxious.
Best of all, my motivation and creative ability have clicked in.
I am an artist—again.
I'm painting every day. It's what is keeping me sane. It's my saving grace.
I'm not perfect. But I am Anne. Again.