Can Genetic Testing Help Shed Light on the Autism Epidemic?
A little boy standing by a window in contemplation. (© altanaka/Fotolia)
Autism cases are still on the rise, and scientists don't know why. In April, the Centers for Disease Control (CDC) reported that rates of autism had increased once again, now at an estimated 1 in 59 children up from 1 in 68 just two years ago. Rates have been climbing steadily since 2007 when the CDC initially estimated that 1 in 150 children were on the autism spectrum.
Some clinicians are concerned that the creeping expansion of autism is causing the diagnosis to lose its meaning.
The standard explanation for this increase has been the expansion of the definition of autism to include milder forms like Asperger's, as well as a heightened awareness of the condition that has improved screening efforts. For example, the most recent jump is attributed to children in minority communities being diagnosed who might have previously gone under the radar. In addition, more federally funded resources are available to children with autism than other types of developmental disorders, which may prompt families or physicians to push harder for a diagnosis.
Some clinicians are concerned that the creeping expansion of autism is causing the diagnosis to lose its meaning. William Graf, a pediatric neurologist at Connecticut Children's Medical Center, says that when a nurse tells him that a new patient has a history of autism, the term is no longer a useful description. "Even though I know this topic extremely well, I cannot picture the child anymore," he says. "Use the words mild, moderate, or severe. Just give me a couple more clues, because when you say autism today, I have no idea what people are talking about anymore."
Genetic testing has emerged as one potential way to remedy the overly broad label by narrowing down a heterogeneous diagnosis to a specific genetic disorder. According to Suma Shankar, a medical geneticist at the University of California, Davis, up to 60 percent of autism cases could be attributed to underlying genetic causes. Common examples include Fragile X Syndrome or Rett Syndrome—neurodevelopmental disorders that are caused by mutations in individual genes and are behaviorally classified as autism.
With more than 500 different mutations associated with autism, very few additional diagnoses provide meaningful information.
Having a genetic diagnosis in addition to an autism diagnosis can help families in several ways, says Shankar. Knowing the genetic origin can alert families to other potential health problems that are linked to the mutation, such as heart defects or problems with the immune system. It may also help clinicians provide more targeted behavioral therapies and could one day lead to the development of drug treatments for underlying neurochemical abnormalities. "It will pave the way to begin to tease out treatments," Shankar says.
When a doctor diagnoses a child as having a specific genetic condition, the label of autism is still kept because it is more well-known and gives the child access to more state-funded resources. Children can thus be diagnosed with multiple conditions: autism spectrum disorder and their specific gene mutation. However, with more than 500 different mutations associated with autism, very few additional diagnoses provide meaningful information. What's more, the presence or absence of a mutation doesn't necessarily indicate whether the child is on the mild or severe end of the autism spectrum.
Because of this, Graf doubts that genetic classifications are really that useful. He tells the story of a boy with epilepsy and severe intellectual disabilities who was diagnosed with autism as a young child. Years later, Graf ordered genetic testing for the boy and discovered that he had a mutation in the gene SYNGAP1. However, this knowledge didn't change the boy's autism status. "That diagnosis [SYNGAP1] turns out to be very specific for him, but it will never be a household name. Biologically it's good to know, and now it's all over his chart. But on a societal level he still needs this catch-all label [of autism]," Graf says.
"It gives some information, but to what degree does that change treatment or prognosis?"
Jennifer Singh, a sociologist at Georgia Tech who wrote the book Multiple Autisms: Spectrums of Advocacy and Genomic Science, agrees. "I don't know that the knowledge gained from just having a gene that's linked to autism," is that beneficial, she says. "It gives some information, but to what degree does that change treatment or prognosis? Because at the end of the day you have to address the issues that are at hand, whatever they might be."
As more children are diagnosed with autism, knowledge of the underlying genetic mutation causing the condition could help families better understand the diagnosis and anticipate their child's developmental trajectory. However, for the vast majority, an additional label provides little clarity or consolation.
Instead of spending money on genetic screens, Singh thinks the resources would be better used on additional services for people who don't have access to behavioral, speech, or occupational therapy. "Things that are really going to matter for this child in their future," she says.
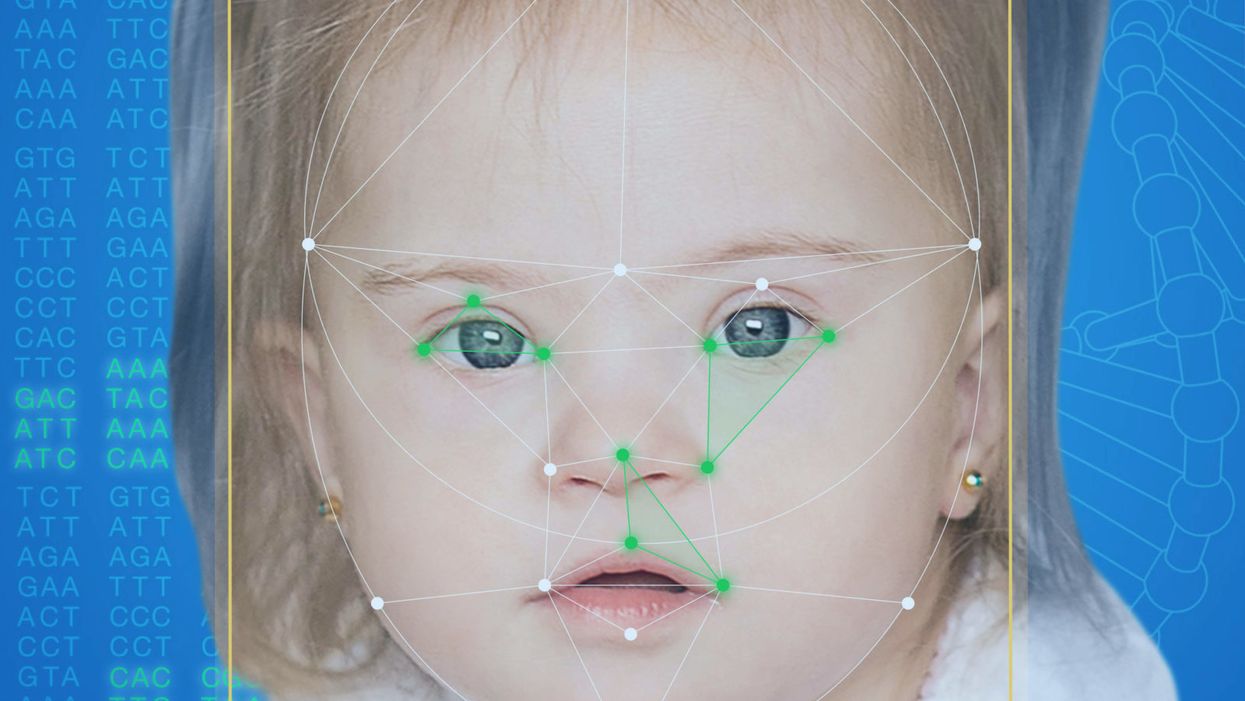
This App Helps Diagnose Rare Genetic Disorders from a Picture
FDNA's Face2Gene technology analyzes patient biometric data using artificial intelligence, identifying correlations with disease-causing genetic variations.
Medical geneticist Omar Abdul-Rahman had a hunch. He thought that the three-year-old boy with deep-set eyes, a rounded nose, and uplifted earlobes might have Mowat-Wilson syndrome, but he'd never seen a patient with the rare disorder before.
"If it weren't for the app I'm not sure I would have had the confidence to say 'yes you should spend $1000 on this test."
Rahman had already ordered genetic tests for three different conditions without any luck, and he didn't want to cost the family any more money—or hope—if he wasn't sure of the diagnosis. So he took a picture of the boy and uploaded the photo to Face2Gene, a diagnostic aid for rare genetic disorders. Sure enough, Mowat-Wilson came up as a potential match. The family agreed to one final genetic test, which was positive for the syndrome.
"If it weren't for the app I'm not sure I would have had the confidence to say 'yes you should spend $1000 on this test,'" says Rahman, who is now the director of Genetic Medicine at the University of Nebraska Medical Center, but saw the boy when he was in the Department of Pediatrics at the University of Mississippi Medical Center in 2012.
"Families who are dealing with undiagnosed diseases never know what's going to come around the corner, what other organ system might be a problem next week," Rahman says. With a diagnosis, "You don't have to wait for the other shoe to drop because now you know the extent of the condition."
A diagnosis is the first and most important step for patients to attain medical care. Disease prognosis, treatment plans, and emotional coping all stem from this critical phase. But diagnosis can also be the trickiest part of the process, particularly for rare disorders. According to one European survey, 40 percent of rare diseases are initially misdiagnosed.
Healthcare professionals and medical technology companies hope that facial recognition software will help prevent families from facing difficult disruptions due to misdiagnoses.
"Patients with rare diseases or genetic disorders go through a long period of diagnostic odyssey, and just putting a name to a syndrome or finding a diagnosis can be very helpful and relieve a lot of tension for the family," says Dekel Gelbman, CEO of FDNA.
Consequently, a misdiagnosis can be devastating for families. Money and time may have been wasted on fruitless treatments, while opportunities for potentially helpful therapies or clinical trials were missed. Parents led down the wrong path must change their expectations of their child's long-term prognosis and care. In addition, they may be misinformed regarding future decisions about family planning.
Healthcare professionals and medical technology companies hope that facial recognition software will help prevent families from facing these difficult disruptions by improving the accuracy and ease of diagnosing genetic disorders. Traditionally, doctors diagnose these types of conditions by identifying unique patterns of facial features, a practice called dysmorphology. Trained physicians can read a child's face like a map and detect any abnormal ridges or plateaus—wide-set eyes, broad forehead, flat nose, rotated ears—that, combined with other symptoms such as intellectual disability or abnormal height and weight, signify a specific genetic disorder.
These morphological changes can be subtle, though, and often only specialized medical geneticists are able to detect and interpret these facial clues. What's more, some genetic disorders are so rare that even a specialist may not have encountered it before, much less a general practitioner. Diagnosing rare conditions has improved thanks to genomic testing that can confirm (or refute) a doctor's suspicion. Yet with thousands of variants in each person's genome, identifying the culprit mutation or deletion can be extremely difficult if you don't know what you're looking for.
Facial recognition technology is trying to take some of the guesswork out of this process. Software such as the Face2Gene app use machine learning to compare a picture of a patient against images of thousands of disorders and come back with suggestions of possible diagnoses.
"This is a classic field for artificial intelligence because no human being can really have enough knowledge and enough experience to be able to do this for thousands of different disorders."
"When we met a geneticist for the first time we were pretty blown away with the fact that they actually use their own human pattern recognition" to diagnose patients, says Gelbman. "This is a classic field for AI [artificial intelligence], for machine learning because no human being can really have enough knowledge and enough experience to be able to do this for thousands of different disorders."
When a physician uploads a photo to the app, they are given a list of different diagnostic suggestions, each with a heat map to indicate how similar the facial features are to a classic representation of the syndrome. The physician can hone the suggestions by adding in other symptoms or family history. Gelbman emphasized that the app is a "search and reference tool" and should not "be used to diagnose or treat medical conditions." It is not approved by the FDA as a diagnostic.
"As a tool, we've all been waiting for this, something that can help everyone," says Julian Martinez-Agosto, an associate professor in human genetics and pediatrics at UCLA. He sees the greatest benefit of facial recognition technology in its ability to empower non-specialists to make a diagnosis. Many areas, including rural communities or resource-poor countries, do not have access to either medical geneticists trained in these types of diagnostics or genomic screens. Apps like Face2Gene can help guide a general practitioner or flag diseases they might not be familiar with.
One concern is that most textbook images of genetic disorders come from the West, so the "classic" face of a condition is often a child of European descent.
Maximilian Muenke, a senior investigator at the National Human Genome Research Institute (NHGRI), agrees that in many countries, facial recognition programs could be the only way for a doctor to make a diagnosis.
"There are only geneticists in countries like the U.S., Canada, Europe, Japan. In most countries, geneticists don't exist at all," Muenke says. "In Nigeria, the most populous country in all of Africa with 160 million people, there's not a single clinical geneticist. So in a country like that, facial recognition programs will be sought after and will be extremely useful to help make a diagnosis to the non-geneticists."
One concern about providing this type of technology to a global population is that most textbook images of genetic disorders come from the West, so the "classic" face of a condition is often a child of European descent. However, the defining facial features of some of these disorders manifest differently across ethnicities, leaving clinicians from other geographic regions at a disadvantage.
"Every syndrome is either more easy or more difficult to detect in people from different geographic backgrounds," explains Muenke. For example, "in some countries of Southeast Asia, the eyes are slanted upward, and that happens to be one of the findings that occurs mostly with children with Down Syndrome. So then it might be more difficult for some individuals to recognize Down Syndrome in children from Southeast Asia."
There is a risk that providing this type of diagnostic information online will lead to parents trying to classify their own children.
To combat this issue, Muenke helped develop the Atlas of Human Malformation Syndromes, a database that incorporates descriptions and pictures of patients from every continent. By providing examples of rare genetic disorders in children from outside of the United States and Europe, Muenke hopes to provide clinicians with a better understanding of what to look for in each condition, regardless of where they practice.
There is a risk that providing this type of diagnostic information online will lead to parents trying to classify their own children. Face2Gene is free to download in the app store, although users must be authenticated by the company as a healthcare professional before they can access the database. The NHGRI Atlas can be accessed by anyone through their website. However, Martinez and Muenke say parents already use Google and WebMD to look up their child's symptoms; facial recognition programs and databases are just an extension of that trend. In fact, Martinez says, "Empowering families is another way to facilitate access to care. Some families live in rural areas and have no access to geneticists. If they can use software to get a diagnosis and then contact someone at a large hospital, it can help facilitate the process."
Martinez also says the app could go further by providing greater transparency about how the program makes its assessments. Giving clinicians feedback about why a diagnosis fits certain facial features would offer a valuable teaching opportunity in addition to a diagnostic aid.
Both Martinez and Muenke think the technology is an innovation that could vastly benefit patients. "In the beginning, I was quite skeptical and I could not believe that a machine could replace a human," says Muenke. "However, I am a convert that it actually can help tremendously in making a diagnosis. I think there is a place for facial recognition programs, and I am a firm believer that this will spread over the next five years."
Why Blindness Will Be the First Disorder Cured by Futuristic Treatments
A blind man with a cane goes for a walk at sunset. (© Prazis/Fotolia)
Stem cells and gene therapy were supposed to revolutionize biomedicine around the turn of the millennium and provide relief for desperate patients with incurable diseases. But for many, progress has been frustratingly slow. We still cannot, for example, regenerate damaged organs like a salamander regrows its tail, and genome engineering is more complicated than cutting and pasting letters in a word document.
"There are a number of things that make [the eye] ideal for new experimental therapies which are not true necessarily in other organs."
For blind people, however, the future of medicine is one step closer to reality. In December, the FDA approved the first gene therapy for an inherited disease—a mutation in the gene RPE65 that causes a rare form of blindness. Several clinical trials also show promise for treating various forms of retinal degeneration using stem cells.
"It's not surprising that the first gene therapy that was approved by the FDA was a therapy in the eye," says Bruce Conklin, a senior investigator at the San Francisco-based Gladstone Institutes, a nonprofit life science research organization, and a professor in the Medical Genetics and Molecular Pharmacology department at the University of California, San Francisco. "There are a number of things that make it ideal for new experimental therapies which are not true necessarily in other organs."
Physicians can easily see into the eye to check if a procedure worked or if it's causing problems. "The imaging technology within the eye is really unprecedented. You can't do this in someone's spinal cord or someone's brain cells or immune system," says Conklin, who is also deputy director of the Innovative Genomics Institute.
There's also a built-in control: researchers can test an intervention on one eye first. What's more, if something goes wrong, the risk of mortality is low, especially when compared to experimenting on the heart or brain. Most types of blindness are currently incurable, so the risk-to-reward ratio for patients is high. If a problem arises with the treatment their eyesight could get worse, but if they do nothing their vision will likely decline anyway. And if the treatment works, they may be able to see for the first time in years.
Gene Therapy
An additional appeal for testing gene therapy in the eye is the low risk for off-target effects, in which genome edits could result in unintended changes to other genes or in other cell types. There are a number of genes that are solely expressed in the eye and not in any other part of the body. Manipulating those genes will only affect cells in the eye, so concerns about the impact on other organs are minimal.
Ninety-three percent of patients who received the injection had improved vision just one month after treatment.
RPE65 is one such gene. It creates an enzyme that helps the eye convert light into an electrical signal that travels back to the brain. Patients with the mutation don't produce the enzyme, so visual signals are not processed. However, the retinal cells in the eye remain healthy for years; if you can restore the missing enzyme you can restore vision.
The newly approved therapy, developed by Spark Therapeutics, uses a modified virus to deliver RPE65 into the eye.A retinal surgeon injects the virus, which has been specially engineered to remove its disease-causing genes and instead carry the correct RPE65 gene, into the retina. There, it is sucked up by retinal pigment epithelial (RPE) cells. The RPE cells are a particularly good target for injection because their job is to eat up and recycle rogue particles. Once inside the cell, the virus slips into the nucleus and releases the DNA. The RPE65 gene then goes to work, using the cell's normal machinery to produce the needed enzyme.
In the most recent clinical trial, 93 percent of patients who received the injection—who range in age from 4 to 44—had improved vision just one month after treatment. So far, the benefits have lasted at least two years.
"It's an exciting time for this class of diseases, where these people have really not had treatments," says Spark president and co-founder, Katherine High. "[Gene therapy] affords the possibility of treatment for diseases that heretofore other classes of therapeutics really have not been able to help."
Stem Cells
Another benefit of the eye is its immune privilege. In order to let light in, the eye must remain transparent. As a result, its immune system is dampened so that it won't become inflamed if outside particles get in. This means the eye is much less likely to reject cell transplants, so patients do not need to take immunosuppressant drugs.
One study generating buzz is a clinical trial in Japan that is the first and, so far, only test of induced pluripotent stem cells in the eye.
Henry Klassen, an assistant professor at UC Irvine, is taking advantage of the eye's immune privilege to transplant retinal progenitor cells into the eye to treat retinitis pigmentosa, an inherited disease affecting about 1 in 4000 people that eventually causes the retina to degenerate. The disease can stem from dozens of different genetic mutations, but the result is the same: RPE cells die off over the course of a few decades, leaving the patient blind by middle age. It is currently incurable.
Retinal progenitor cells are baby retinal cells that develop naturally from stem cells and will turn into one of several types of adult retinal cells. When transplanted into a patient's eye, the progenitor cells don't replace the lost retinal cells, but they do secrete proteins and enzymes essential for eye health.
"At the stage we get the retinal tissue it's immature," says Klassen. "They still have some flexibility in terms of which mature cells they can turn into. It's that inherent flexibility that gives them a lot of power when they're put in the context of a diseased retina."
Klassen's spin-off company, jCyte, sponsored the clinical trial with support from the California Institute for Regenerative Medicine. The results from the initial study haven't been published yet, but Klassen says he considers it a success. JCyte is now embarking on a phase two trial to assess improvements in vision after the treatment, which will wrap up in 2021.
Another study generating buzz is a clinical trial in Japan that is the first and, so far, only test of induced pluripotent stem cells (iPSC) in the eye. iPSC are created by reprogramming a patient's own skin cells into stem cells, circumventing any controversy around embryonic stem cell sources. In the trial, led by Masayo Takahashi at RIKEN, the scientists transplant retinal pigment epithelial cells created from iPSC into the retinas of patients with age-related macular degeneration. The first woman to receive the treatment is doing well, and her vision is stable. However, the second patient suffered a swollen retina as a result of the surgery. Despite this recent setback, Takahashi said last week that the trial would continue.
Botched Jobs
Although recent studies have provided patients with renewed hope, the field has not been without mishap. Most notably, an article in the New England Journal of Medicine last March described three patients who experienced severe side effects after receiving stem cell injections from a Florida clinic to treat age-related macular degeneration. Following the initial article, other reports came out about similar botched treatments. Lawsuits have been filed against US Stem Cell, the clinic that conducted the procedure, and the FDA sent them a warning letter with a long list of infractions.
"One red flag is that the clinics charge patients to take part in the treatment—something extremely unusual for legitimate clinical trials."
Ajay Kuriyan, an ophthalmologist and retinal specialist at the University of Rochester who wrote the paper, says that because details about the Florida trial are scarce, it's hard to say why the treatment caused the adverse reaction. His guess is that the stem cells were poorly prepared and not up to clinical standards.
Klassen agrees that small clinics like US Stem Cell do not offer the same caliber of therapy as larger clinical trials. "It's not the same cells and it's not the same technique and it's not the same supervision and it's not under FDA auspices. It's just not the same thing," he says. "Unfortunately, to the patient it might sound the same, and that's the tragedy for me."
For patients who are interested in joining a trial, Kuriyan listed a few things to watch out for. "One red flag is that the clinics charge patients to take part in the treatment—something extremely unusual for legitimate clinical trials," he says. "Another big red flag is doing the procedure in both eyes" at the same time. Third, if the only treatment offered is cell therapy. "These clinics tend to be sort of stand-alone clinics, and that's not very common for an actual big research study of this scale."
Despite the recent scandal, Klassen hopes that the success of his trial and others will continue to push the field forward. "It just takes so many decades to move this stuff along, even when you're trying to simplify it as much as possible," he says. "With all the heavy lifting that's been done, I hope the world's got the patience to get this through."