Study Shows “Living Drug” Can Provide a Lasting Cure for Cancer

A recent study by researchers at the University of Pennsylvania examined how CAR-T therapy helped Doug Olson beat a cancer death sentence for over a decade - and how it could work for more people.
Doug Olson was 49 when he was diagnosed with chronic lymphocytic leukemia, a blood cancer that strikes 21,000 Americans annually. Although the disease kills most patients within a decade, Olson’s case progressed more slowly, and courses of mild chemotherapy kept him healthy for 13 years. Then, when he was 62, the medication stopped working. The cancer had mutated, his doctor explained, becoming resistant to standard remedies. Harsher forms of chemo might buy him a few months, but their side effects would be debilitating. It was time to consider the treatment of last resort: a bone-marrow transplant.
Olson, a scientist who developed blood-testing instruments, knew the odds. There was only a 50 percent chance that a transplant would cure him. There was a 20 percent chance that the agonizing procedure—which involves destroying the patient’s marrow with chemo and radiation, then infusing his blood with donated stem cells—would kill him. If he survived, he would face the danger of graft-versus-host disease, in which the donor’s cells attack the recipient’s tissues. To prevent it, he would have to take immunosuppressant drugs, increasing the risk of infections. He could end up with pneumonia if one of his three grandchildren caught a sniffle. “I was being pushed into a corner,” Olson recalls, “with very little room to move.”
Soon afterward, however, his doctor revealed a possible escape route. He and some colleagues at the University of Pennsylvania’s Abramson Cancer Center were starting a clinical trial, he said, and Olson—still mostly symptom-free—might be a good candidate. The experimental treatment, known as CAR-T therapy, would use genetic engineering to turn his T lymphocytes (immune cells that guard against viruses and other pathogens) into a weapon against cancer.
In September 2010, technicians took some of Olson’s T cells to a laboratory, where they were programmed with new molecular marching orders and coaxed to multiply into an army of millions. When they were ready, a nurse inserted a catheter into his neck. At the turn of a valve, his soldiers returned home, ready to do battle.
“I felt like I’d won the lottery,” Olson says. But he was only the second person in the world to receive this “living drug,” as the University of Pennsylvania investigators called it. No one knew how long his remission would last.
Three weeks later, Olson was slammed with a 102-degree fever, nausea, and chills. The treatment had triggered two dangerous complications: cytokine release syndrome, in which immune chemicals inflame the patient’s tissues, and tumor lysis syndrome, in which toxins from dying cancer cells overwhelm the kidneys. But the crisis passed quickly, and the CAR-T cells fought on. A month after the infusion, the doctor delivered astounding news: “We can’t find any cancer in your body.”
“I felt like I’d won the lottery,” Olson says. But he was only the second person in the world to receive this “living drug,” as the University of Pennsylvania investigators called it. No one knew how long his remission would last.
An Unexpected Cure
In February 2022, the same cancer researchers reported a remarkable milestone: the trial’s first two patients had survived for more than a decade. Although Olson’s predecessor—a retired corrections officer named Bill Ludwig—died of COVID-19 complications in early 2021, both men had remained cancer-free. And the modified immune cells continued to patrol their territory, ready to kill suspected tumor cells the moment they arose.
“We can now conclude that CAR-T cells can actually cure patients with leukemia,” University of Pennsylvania immunologist Carl June, who spearheaded the development of the technique, told reporters. “We thought the cells would be gone in a month or two. The fact that they’ve survived 10 years is a major surprise.”
Even before the announcement, it was clear that CAR-T therapy could win a lasting reprieve for many patients with cancers that were once a death sentence. Since the Food and Drug Administration approved June’s version (marketed as Kymriah) in 2017, the agency has greenlighted five more such treatments for various types of leukemia, lymphoma, and myeloma. “Every single day, I take care of patients who would previously have been told they had no options,” says Rayne Rouce, a pediatric hematologist/oncologist at Texas Children’s Cancer Center. “Now we not only have a treatment option for those patients, but one that could potentially be the last therapy for their cancer that they’ll ever have to receive.”

Immunologist Carl June, middle, spearheaded development of the CAR-T therapy that gave patients Bill Ludwig, left, and Doug Olson, right, a lengthy reprieve on their terminal cancer diagnoses.
Penn Medicine
Yet the CAR-T approach doesn’t help everyone. So far, it has only shown success for blood cancers—and for those, the overall remission rate is 30 to 40 percent. “When it works, it works extraordinarily well,” says Olson’s former doctor, David Porter, director of Penn’s blood and bone marrow transplant program. “It’s important to know why it works, but it’s equally important to know why it doesn’t—and how we can fix that.”
The team’s study, published in the journal Nature, offers a wealth of data on what worked for these two patients. It may also hold clues for how to make the therapy effective for more people.
Building a Better T Cell
Carl June didn’t set out to cure cancer, but his serendipitous career path—and a personal tragedy—helped him achieve insights that had eluded other researchers. In 1971, hoping to avoid combat in Vietnam, he applied to the U.S. Naval Academy in Annapolis, Maryland. June showed a knack for biology, so the Navy sent him on to Baylor College of Medicine. He fell in love with immunology during a fellowship researching malaria vaccines in Switzerland. Later, the Navy deployed him to the Fred Hutchinson Cancer Research Center in Seattle to study bone marrow transplantation.
There, June became part of the first research team to learn how to culture T cells efficiently in a lab. After moving on to the National Naval Medical Center in the ’80s, he used that knowledge to combat the newly emerging AIDS epidemic. HIV, the virus that causes the disease, invades T cells and eventually destroys them. June and his post-doc Bruce Levine developed a method to restore patients’ depleted cell populations, using tiny magnetic beads to deliver growth-stimulating proteins. Infused into the body, the new T cells effectively boosted immune function.
In 1999, after leaving the Navy, June joined the University of Pennsylvania. His wife, who’d been diagnosed with ovarian cancer, died two years later, leaving three young children. “I had not known what it was like to be on the other side of the bed,” he recalls. Watching her suffer through grueling but futile chemotherapy, followed by an unsuccessful bone-marrow transplant, he resolved to focus on finding better cancer treatments. He started with leukemia—a family of diseases in which mutant white blood cells proliferate in the marrow.
Cancer is highly skilled at slipping through the immune system’s defenses. T cells, for example, detect pathogens by latching onto them with receptors designed to recognize foreign proteins. Leukemia cells evade detection, in part, by masquerading as normal white blood cells—that is, as part of the immune system itself.
June planned to use a viral vector no one had tried before: HIV.
To June, chimeric antigen receptor (CAR) T cells looked like a promising tool for unmasking and destroying the impostors. Developed in the early ’90s, these cells could be programmed to identify a target protein, and to kill any pathogen that displayed it. To do the programming, you spliced together snippets of DNA and inserted them into a disabled virus. Next, you removed some of the patient’s T cells and infected them with the virus, which genetically hijacked its new hosts—instructing them to find and slay the patient’s particular type of cancer cells. When the T cells multiplied, their descendants carried the new genetic code. You then infused those modified cells into the patient, where they went to war against their designated enemy.
Or that’s what happened in theory. Many scientists had tried to develop therapies using CAR-T cells, but none had succeeded. Although the technique worked in lab animals, the cells either died out or lost their potency in humans.
But June had the advantage of his years nurturing T cells for AIDS patients, as well as the technology he’d developed with Levine (who’d followed him to Penn with other team members). He also planned to use a viral vector no one had tried before: HIV, which had evolved to thrive in human T cells and could be altered to avoid causing disease. By the summer of 2010, he was ready to test CAR-T therapy against chronic lymphocytic leukemia (CLL), the most common form of the disease in adults.
Three patients signed up for the trial, including Doug Olson and Bill Ludwig. A portion of each man’s T cells were reprogrammed to detect a protein found only on B lymphocytes, the type of white blood cells affected by CLL. Their genetic instructions ordered them to destroy any cell carrying the protein, known as CD19, and to multiply whenever they encountered one. This meant the patients would forfeit all their B cells, not just cancerous ones—but regular injections of gamma globulins (a cocktail of antibodies) would make up for the loss.
After being infused with the CAR-T cells, all three men suffered high fevers and potentially life-threatening inflammation, but all pulled through without lasting damage. The third patient experienced a partial remission and survived for eight months. Olson and Ludwig were cured.
Learning What Works
Since those first infusions, researchers have developed reliable ways to prevent or treat the side effects of CAR-T therapy, greatly reducing its risks. They’ve also been experimenting with combination therapies—pairing CAR-T with chemo, cancer vaccines, and immunotherapy drugs called checkpoint inhibitors—to improve its success rate. But CAR-T cells are still ineffective for at least 60 percent of blood cancer patients. And they remain in the experimental stage for solid tumors (including pancreatic cancer, mesothelioma, and glioblastoma), whose greater complexity make them harder to attack.
The new Nature study offers clues that could fuel further advances. The Penn team “profiled these cells at a level where we can almost say, ‘These are the characteristics that a T cell would need to survive 10 years,’” says Rouce, the physician at Texas Children’s Cancer Center.
One surprising finding involves how CAR-T cells change in the body over time. At first, those that Olson and Ludwig received showed the hallmarks of “killer” T-cells (also known as CD8 cells)—highly active lymphocytes bent on exterminating every tumor cell in sight. After several months, however, the population shifted toward “helper” T-cells (or CD4s), which aid in forming long-term immune memory but are normally incapable of direct aggression. Over the years, the numbers swung back and forth, until only helper cells remained. Those cells showed markers suggesting they were too exhausted to function—but in the lab, they were able not only to recognize but to destroy cancer cells.
June and his team suspect that those tired-looking helper cells had enough oomph to kill off any B cells Olson and Ludwig made, keeping the pair’s cancers permanently at bay. If so, that could prompt new approaches to selecting cells for CAR-T therapy. Maybe starting with a mix of cell types—not only CD8s, but CD4s and other varieties—would work better than using CD8s alone. Or perhaps inducing changes in cell populations at different times would help.
Another potential avenue for improvement is starting with healthier cells. Evidence from this and other trials hints that patients whose T cells are more robust to begin with respond better when their cells are used in CAR-T therapy. The Penn team recently completed a clinical trial in which CLL patients were treated with ibrutinib—a drug that enhances T-cell function—before their CAR-T cells were manufactured. The response rate, says David Porter, was “very high,” with most patients remaining cancer-free a year after being infused with the souped-up cells.
Such approaches, he adds, are essential to achieving the next phase in CAR-T therapy: “Getting it to work not just in more people, but in everybody.”

Doug Olson enjoys nature - and having a future.
Penn Medicine
To grasp what that could mean, it helps to talk with Doug Olson, who’s now 75. In the years since his infusion, he has watched his four children forge careers, and his grandkids reach their teens. He has built a business and enjoyed the rewards of semi-retirement. He’s done volunteer and advocacy work for cancer patients, run half-marathons, sailed the Caribbean, and ridden his bike along the sun-dappled roads of Silicon Valley, his current home.
And in his spare moments, he has just sat there feeling grateful. “You don’t really appreciate the effect of having a lethal disease until it’s not there anymore,” he says. “The world looks different when you have a future.”
This article was first published on Leaps.org on March 24, 2022.
Inside Scoop: How a DARPA Scientist Helped Usher in a Game-Changing Covid Treatment
Amy Jenkins is a program manager for the Defense Advanced Research Projects Agency's Biological Technologies Office, which runs a project called the Pandemic Prevention Platform.
Amy Jenkins was in her office at DARPA, a research and development agency within the Department of Defense, when she first heard about a respiratory illness plaguing the Chinese city of Wuhan. Because she's a program manager for DARPA's Biological Technologies Office, her colleagues started stopping by. "It's really unusual, isn't it?" they would say.
At the time, China had a few dozen cases of what we now call COVID-19. "We should maybe keep an eye on that," she thought.
Early in 2020, still just keeping watch, she was visiting researchers working on DARPA's Pandemic Prevention Platform (P3), a project to develop treatments for "any known or previously unknown infectious threat," within 60 days of its appearance. "We looked at each other and said, 'Should we be doing something?'" she says.
For projects like P3, groups of scientists—often at universities and private companies—compete for DARPA contracts, and program managers like Jenkins oversee the work. Those that won the P3 bid included scientists at AbCellera Biologics, Inc., AstraZeneca, Duke University, and Vanderbilt University.
At the time Jenkins was talking to the P3 performers, though, they didn't have evidence of community transmission. "We would have to cross that bar before we considered doing anything," she says.
The world soon leapt far over that bar. By the time Jenkins and her team decided P3 should be doing something—with their real work beginning in late February--it was too late to prevent this pandemic. But she could help P3 dig into the chemical foundations of COVID-19's malfeasance, and cut off its roots. That work represents, in fact, her roots.
In late February 2020, DARPA received a single blood sample from a recovered COVID-19 patient, in which P3 researchers could go fishing for antibodies. The day it arrived, Jenkins's stomach roiled. "We get one shot," she thought.
Fighting the Smallest Enemies
Jenkins, who's in her early 40s, first got into germs the way many 90s kids did: by reading The Hot Zone, a novel about a hemorrhagic fever gone rogue. It wasn't exactly the disintegrating organs that hooked her. It was the idea that "these very pathogens that we can't even see can make us so sick and bring us to our knees," she says. Reading about scientists facing down deadly disease, she wondered, "How do these things make you so sick?"
She chased that question in college, majoring in both biomolecular science and chemistry, and later became an antibody expert. Antibodies are proteins that hook to a pathogen to block it from attaching to your cells, or tag it for destruction by the rest of the immune system. Soon, she jumped on the "monoclonal antibodies" train—developing synthetic versions of these natural defenses, which doctors can give to people to help them battle an early-stage infection, and even to prevent an infection from taking root after an exposure.
Jenkins likens the antibody treatments to the old aphorism about fishing: Vaccines teach your body how to fish, but antibodies simply give your body the pesca-fare. While that, as the saying goes, won't feed you for a lifetime, it will last a few weeks or months. Monoclonal antibodies thus are a promising preventative option in the immediate short-term when a vaccine hasn't yet been given (or hasn't had time to produce an immune response), as well as an important treatment weapon in the current fight. After former president Donald Trump contracted COVID-19, he received a monoclonal antibody treatment from biotech company Regeneron.
As for Jenkins, she started working as a DARPA Biological Technologies Office contractor soon after completing her postdoc. But it was a suit job, not a labcoat job. And suit jobs, at first, left Jenkins conflicted, worried about being bored. She'd give it a year, she thought. But the year expired, and bored she was not. Around five years later, in June 2019, the agency hired her to manage several of the office's programs. A year into that gig, the world was months into a pandemic.
The Pandemic Pivot
At DARPA, Jenkins inherited five programs, including P3. P3 works by taking blood from recovered people, fishing out their antibodies, identifying the most effective ones, and then figuring out how to manufacture them fast. Back then, P3 existed to help with nebulous, future outbreaks: Pandemic X. Not this pandemic. "I did not have a crystal ball," she says, "but I will say that all of us in the infectious diseases and public-health realm knew that the next pandemic was coming."
Three days after a January 2020 meeting with P3 researchers, COVID-19 appeared in Seattle, then began whipping through communities. The time had come for P3 teams to swivel. "We had done this," she says. "We had practiced this before." But would their methods stand up to something unknown, racing through the global population? "The big anxiety was, 'Wow, this was real,'" says Jenkins.
While facing down that realness, Jenkins was also managing other projects. In one called PREPARE, groups develop "medical countermeasures" that modulate a person's genetic code to boost their bodies' responses to threats. Another project, NOW, envisions shipping-container-sized factories that can make thousands of vaccine doses in days. And then there's Prometheus—which means "forethought" in Greek, and is the name of the god who stole fire and gave it to humans. Wrapping up as COVID ramped up, Prometheus aimed to identify people who are contagious—with whatever—before they start coughing, and even if they never do.
All of DARPA's projects focus on developing early-stage technology, passing it off to other agencies or industry to put it into operation. The orientation toward a specific goal appealed to Jenkins, as a contrast to academia. "You go down a rabbit hole for years at a time sometimes, chasing some concept you found interesting in the lab," she says. That's good for the human pursuit of knowledge, and leads to later applications, but DARPA wants a practical prototype—stat.
"Dual-Use" Technologies
That desire, though, and the fact that DARPA is a defense agency, present philosophical complications. "Bioethics in the national-security context turns all the dials up to 10+," says Jonathan Moreno, a medical ethicist at the University of Pennsylvania.
While developing antibody treatments to stem a pandemic seems straightforwardly good, all biological research—especially that backed by military money—requires evaluating potential knock-on applications, even those that might come from outside the entity that did the developing. As Moreno put it, "Albert Einstein wasn't thinking about blowing up Hiroshima." Particularly sensitive are so-called "dual-use" technologies—those tools that could be used for both benign and nefarious purposes, or are of interest to both the civilian and military worlds.
Moreno takes Prometheus itself as an example of "dual-use" technology. "Think about somebody wearing a suicide vest. Instead of a suicide vest, make them extremely contagious with something. The flu plus Ebola," he says. "Send them someplace, a sensitive environment. We would like to be able to defend against that"—not just tell whether Uncle Fred is bringing asymptomatic COVID home for Christmas. Prometheus, Jenkins says, had safety in mind from the get-go, and required contenders to "develop a risk mitigation plan" and "detail their strategy for appropriate control of information."
To look at a different program, if you can modulate genes to help healing, you probably know something (or know someone else could infer something) about how to hinder healing. Those sorts of risks are why PREPARE researchers got their own "ethical, legal, and social implications" panel, which meets quarterly "to ensure that we are performing all research and publications in a safe and ethical manner," says Jenkins.
DARPA as a whole, Moreno says, is institutionally sensitive to bioethics. The agency has ethics panels, and funded a 2014 National Academies assessment of how to address the "ethical, legal, and societal issues" around technology that has military relevance. "In the cases of biotechnologies where some of that research brushes up against what could legitimately be considered dual-use, that in itself justifies our investment," says Jenkins. "DARPA deliberately focuses on safety and countermeasures against potentially dangerous technologies, and we structure our programs to be transparent, safe, and legal."
Going Fishing
In late February 2020, DARPA received a single blood sample from a recovered COVID-19 patient, in which P3 researchers could go fishing for antibodies. The day it arrived, Jenkins's stomach roiled. "We get one shot," she thought.
As scientists from the P3-funded AbCellera went through the processes they'd practiced, Jenkins managed their work, tracking progress and relaying results. Soon, the team had isolated a suitable protein: bamlanivimab. It attaches to and blocks off the infamous spike proteins on SARS-CoV-2—those sticky suction-cups in illustrations. Partnering with Eli Lilly in a manufacturing agreement, the biotech company brought it to clinical trials in May, just a few months after its work on the deadly pathogen began, after much of the planet became a hot zone.
On November 10—Jenkins's favorite day at the (home) office—the FDA provided Eli Lilly emergency use authorization for bamlanivimab. But she's only mutedly screaming (with joy) inside her heart. "This pandemic isn't 'one morning we're going to wake up and it's all over,'" she says. When it is over, she and her colleagues plan to celebrate their promethean work. "I'm hoping to be able to do it in person," she says. "Until then, I have not taken a breath."
Everyone Should Hear My COVID Vaccine Experience
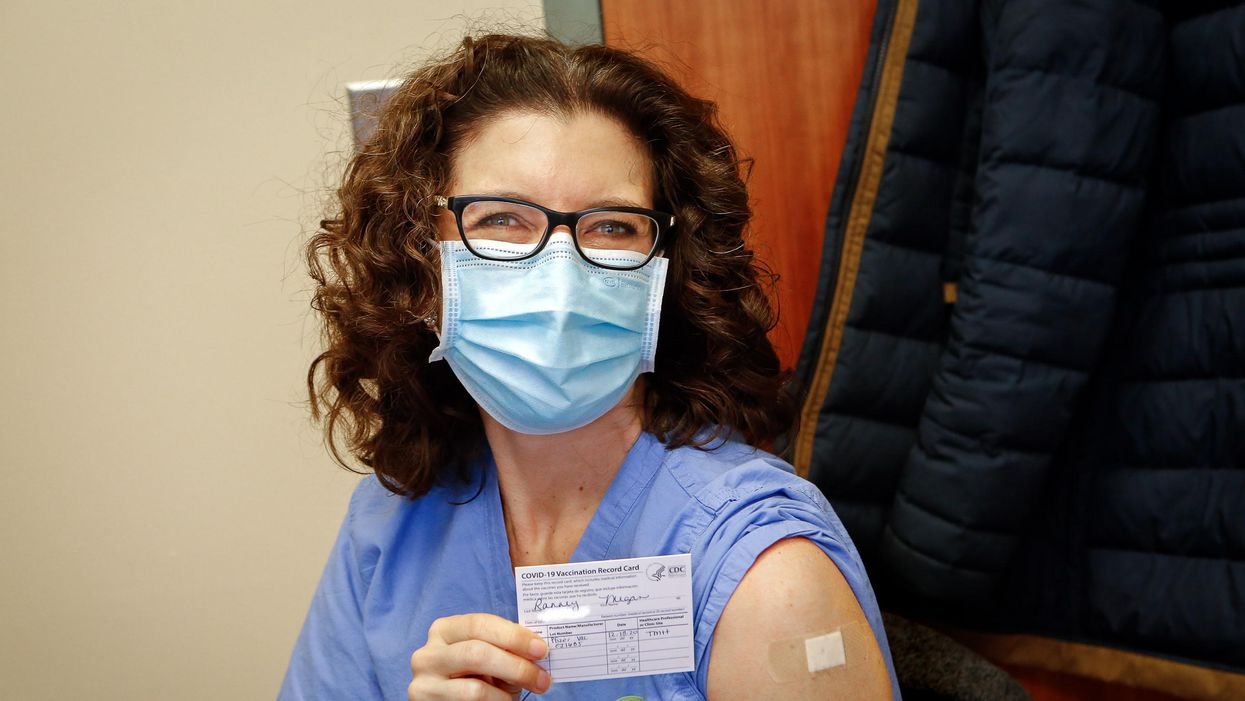
Dr. Ranney immediately after receiving her first dose of the Pfizer vaccine on December 18, 2020.
On December 18th, 2020, I received my first dose of the Pfizer mRNA vaccine against SARS-CoV-2. On January 9th, 2021, I received my second. I am now a CDC-card-carrying, fully vaccinated person.
The build-up to the first dose was momentous. I was scheduled for the first dose of the morning. Our vaccine clinic was abuzz with excitement and hope, and some media folks were there to capture the moment. A couple of fellow emergency physicians were in the same cohort of recipients as I; we exchanged virtual high-fives and took a picture of socially distanced hugs. It was, after all, the closest thing we'd had to a celebration in months.
I walked in the vaccine administration room with anticipation – it was tough to believe this moment was truly, finally here. I got a little video of my getting the shot, took my obligate vaccine selfie, waited in the observation area for 15 minutes to ensure I didn't have a reaction, and then proudly joined 1000s of fellow healthcare workers across the country in posting #ThisIsMyShot on social media. "Here we go, America!"
The first shot, though, didn't actually do all that much for me. It hurt less than a flu shot (which, by the way, doesn't hurt much). I had virtually no side effects. I also knew that it did not yet protect me. The Pfizer (and Moderna) data show very clearly that although the immune response starts to grow 10-12 days after the first shot, one doesn't reach full protection against COVID-19 until much later.
So when, two days after my first shot, I headed back to work in the emergency department, I kept wondering "Will this be the day that I get sick? Wouldn't that be ironic!" Although I never go without an N95 during patient care, it just takes one slip – scratching one's eyes, eating lunch in a break room that an infected colleague had just been in – to get ill. Ten months into this pandemic, it is so easy to get fatigued, to make a small error just one time.
Indeed, I had a few colleagues fall ill in between their first and second shots; one was hospitalized. This was not surprising, but still sad, given how close they had come to escaping infection.
Scientifically speaking, one doesn't need to feel bad to develop an immune response. Emotionally, though, I welcomed the symptoms as proof positive that I would be protected.
This time period felt a little like we had our learner's permit for driving: we were on our way to being safe, but not quite there yet.
I also watched, with dismay, our failures as a nation at timely distribution of the vaccine. On December 18th, despite the logistical snafus that many of us had started to highlight, it was still somewhat believable that we would at least distribute (if not actually administer) 20 million doses by the New Year. But by December 31, my worst fears about the feds' lack of planning had been realized. Only 14 million doses had gone to states, and fewer than 3 million had been administered. Within the public health and medical community, we began to debate how to handle the shortages and slow vaccination rates: should we change prioritization schemes? Get rid of the second dose, in contradiction to what our FDA had approved?
Let me be clear: I really, really, really wanted my second dose. It is what is supported by the data. After living this long at risk, it felt frankly unfair that I might not get fully protected. I waited with trepidation, afraid that policies would shift before I got it in my arm.
At last, my date for my second shot arrived.
This shot was a little less momentous on the outside. The vaccine clinic was much more crowded, as we were now administering first doses to more people, as well as providing the second dose to many. There were no high fives, no media, and I took no selfies. I finished my observation period without trouble (as did everyone else vaccinated the same day, as is typical for these vaccines). I walked out the door planning to spend a nice afternoon outdoors with my kids.
Within 15 minutes, though, the very common side effects – reported by 80% of people my age after the second dose – began to appear. First I got a headache (like 52% of people my age), then body aches (37%), fatigue (59%), and chills (35%). I felt "foggy", like I was fighting something. Like 45% of trial participants who had received the actual vaccine, I took acetaminophen and ibuprofen to stave off the symptoms. There is some minimal evidence from other vaccines that pre-treatment with these anti-inflammatories may reduce antibodies, but given that half of trial participants took these medications, there's no reason to make yourself suffer if you develop side effects. Forty-eight hours later, just in time for my next shift, the side effects magically cleared. Scientifically speaking, one doesn't need to feel bad to develop an immune response. Emotionally, though, I welcomed the symptoms as proof positive that I would be protected.
My reaction was truly typical. Although the media hype focuses on major negative reactions, they are – statistically speaking – tremendously rare: fewer than 11/million people who received the Pfizer vaccine, and 3/million who received the Moderna vaccine, developed anaphylaxis; of these, all were treated, and all are fine. Compare this with the fact that approximately 1200/million Americans have died of this virus. I'll choose the minor, temporary, utterly treatable side effects any day.
Now, more than 14 days after my second dose, the data says that my chance of getting really sick is, truly, infinitesimally low. I don't have to worry that each shift will put me into the hospital. I feel emotionally lighter, and a little bit like I have a secret super-power.
But I also know that we are not yet home free.
I may have my personal equivalent of Harry Potter's invisibility cloak – but we don't yet know whether it protects those around me, at all. As Dr. Fauci himself has written, while community spread is high, there is still a chance that I could be a carrier of infection to others. So I still wear my N95 at work, I still mask in public, and I still shower as soon as I get home from a shift and put my scrubs right in the washing machine to protect my husband and children. I also won't see my parents indoors until they, too, have been vaccinated.
At the end of the day, these vaccines are both amazing and life-changing, and not. My colleagues are getting sick less often, now that many of us are a week or more out from our second dose. I can do things (albeit still masked) that would simply not have been safe a month ago. These are small miracles, for which I am thankful. But like so many things in life, they would be better if shared with others. Only when my community is mostly vaccinated, will I breathe easy again.
My deepest hope is that we all have – and take - the chance to get our shots, soon. Because although the symbolism and effect of the vaccine is high, the experience itself was … not that big a deal.