Researchers advance drugs that treat pain without addiction
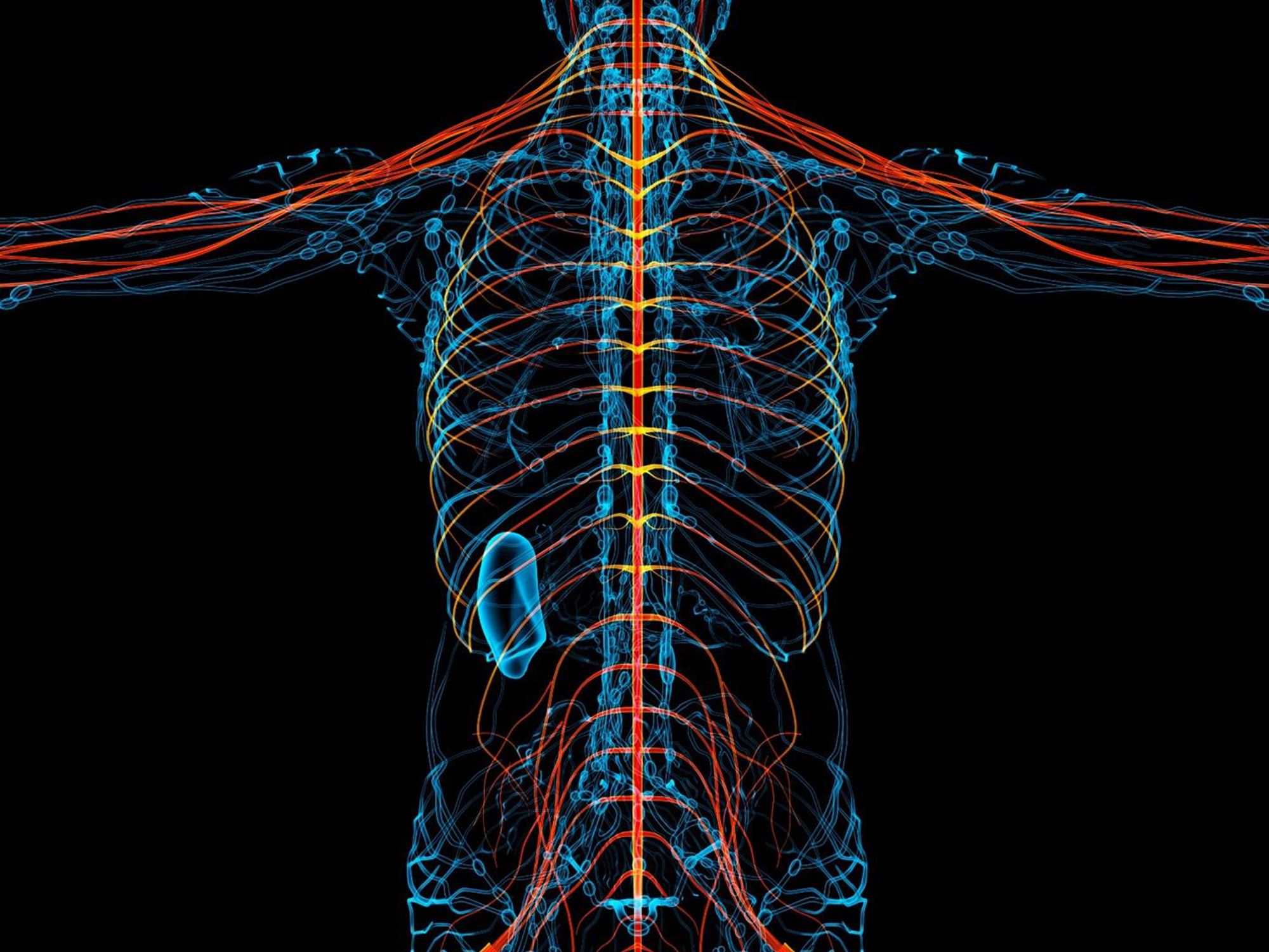
New therapies are using creative approaches that target the body’s sensory neurons, which send pain signals to the brain.
Opioids are one of the most common ways to treat pain. They can be effective but are also highly addictive, an issue that has fueled the ongoing opioid crisis. In 2020, an estimated 2.3 million Americans were dependent on prescription opioids.
Opioids bind to receptors at the end of nerve cells in the brain and body to prevent pain signals. In the process, they trigger endorphins, so the brain constantly craves more. There is a huge risk of addiction in patients using opioids for chronic long-term pain. Even patients using the drugs for acute short-term pain can become dependent on them.
Scientists have been looking for non-addictive drugs to target pain for over 30 years, but their attempts have been largely ineffective. “We desperately need alternatives for pain management,” says Stephen E. Nadeau, a professor of neurology at the University of Florida.
A “dimmer switch” for pain
Paul Blum is a professor of biological sciences at the University of Nebraska. He and his team at Neurocarrus have created a drug called N-001 for acute short-term pain. N-001 is made up of specially engineered bacterial proteins that target the body’s sensory neurons, which send pain signals to the brain. The proteins in N-001 turn down pain signals, but they’re too large to cross the blood-brain barrier, so they don’t trigger the release of endorphins. There is no chance of addiction.
When sensory neurons detect pain, they become overactive and send pain signals to the brain. “We wanted a way to tone down sensory neurons but not turn them off completely,” Blum reveals. The proteins in N-001 act “like a dimmer switch, and that's key because pain is sensation overstimulated.”
Blum spent six years developing the drug. He finally managed to identify two proteins that form what’s called a C2C complex that changes the structure of a subunit of axons, the parts of neurons that transmit electrical signals of pain. Changing the structure reduces pain signaling.
“It will be a long path to get to a successful clinical trial in humans," says Stephen E. Nadeau, professor of neurology at the University of Florida. "But it presents a very novel approach to pain reduction.”
Blum is currently focusing on pain after knee and ankle surgery. Typically, patients are treated with anesthetics for a short time after surgery. But anesthetics usually only last for 4 to 6 hours, and long-term use is toxic. For some, the pain subsides. Others continue to suffer after the anesthetics have worn off and start taking opioids.
N-001 numbs sensation. It lasts for up to 7 days, much longer than any anesthetic. “Our goal is to prolong the time before patients have to start opioids,” Blum says. “The hope is that they can switch from an anesthetic to our drug and thereby decrease the likelihood they're going to take the opioid in the first place.”
Their latest animal trial showed promising results. In mice, N-001 reduced pain-like behaviour by 90 percent compared to the control group. One dose became effective in two hours and lasted a week. A high dose had pain-relieving effects similar to an opioid.
Professor Stephen P. Cohen, director of pain operations at John Hopkins, believes the Neurocarrus approach has potential but highlights the need to go beyond animal testing. “While I think it's promising, it's an uphill battle,” he says. “They have shown some efficacy comparable to opioids, but animal studies don't translate well to people.”
Nadeau, the University of Florida neurologist, agrees. “It will be a long path to get to a successful clinical trial in humans. But it presents a very novel approach to pain reduction.”
Blum is now awaiting approval for phase I clinical trials for acute pain. He also hopes to start testing the drug's effect on chronic pain.
Learning from people who feel no pain
Like Blum, a pharmaceutical company called Vertex is focusing on treating acute pain after surgery. But they’re doing this in a different way, by targeting a sodium channel that plays a critical role in transmitting pain signals.
In 2004, Stephen Waxman, a neurology professor at Yale, led a search for genetic pain anomalies and found that biologically related people who felt no pain despite fractures, burns and even childbirth had mutations in the Nav1.7 sodium channel. Further studies in other families who experienced no pain showed similar mutations in the Nav1.8 sodium channel.
Scientists set out to modify these channels. Many unsuccessful efforts followed, but Vertex has now developed VX-548, a medicine to inhibit Nav1.8. Typically, sodium ions flow through sodium channels to generate rapid changes in voltage which create electrical pulses. When pain is detected, these pulses in the Nav1.8 channel transmit pain signals. VX-548 uses small molecules to inhibit the channel from opening. This blocks the flow of sodium ions and the pain signal. Because Nav1.8 operates only in peripheral nerves, located outside the brain, VX-548 can relieve pain without any risk of addiction.
"Frankly we need drugs for chronic pain more than acute pain," says Waxman.
The team just finished phase II clinical trials for patients following abdominoplasty surgery and bunionectomy surgery.
After abdominoplasty surgery, 76 patients were treated with a high dose of VX-548. Researchers then measured its effectiveness in reducing pain over 48 hours, using the SPID48 scale, in which higher scores are desirable. The score for Vertex’s drug was 110.5 compared to 72.7 in the placebo group, whereas the score for patients taking an opioid was 85.2. The study involving bunionectomy surgery showed positive results as well.
Waxman, who has been at the forefront of studies into Nav1.7 and Nav1.8, believes that Vertex's results are promising, though he highlights the need for further clinical trials.
“Blocking Nav1.8 is an attractive target,” he says. “[Vertex is] studying pain that is relatively simple and uniform, and that's key to having a drug trial that is informative. But the study needs to be replicated and frankly we need drugs for chronic pain more than acute pain. If this is borne out by additional studies, it's one important step in a journey.”
Vertex will be launching phase III trials later this year.
Finding just the right amount of Nerve Growth Factor
Whereas Neurocarrus and Vertex are targeting short-term pain, a company called Levicept is concentrating on relieving chronic osteoarthritis pain. Around 32.5 million Americans suffer from osteoarthritis. Patients commonly take NSAIDs, or non-steroidal anti-inflammatory drugs, but they cannot be taken long-term. Some take opioids but they aren't very effective.
Levicept’s drug, Levi-04, is designed to modify a signaling pathway associated with pain. Nerve Growth Factor (NGF) is a neurotrophin: it’s involved in nerve growth and function. NGF signals by attaching to receptors. In pain there are excess neurotrophins attaching to receptors and activating pain signals.
“What Levi-04 does is it returns the natural equilibrium of neurotrophins,” says Simon Westbrook, the CEO and founder of Levicept. It stabilizes excess neurotrophins so that the NGF pathway does not signal pain. Levi-04 isn't addictive since it works within joints and in nerves outside the brain.
Westbrook was initially involved in creating an anti-NGF molecule for Pfizer called Tanezumab. At first, Tanezumab seemed effective in clinical trials and other companies even started developing their own versions. However, a problem emerged. Tanezumab caused rapidly progressive osteoarthritis, or RPOA, in some patients because it completely removed NGF from the system. NGF is not just involved in pain signalling, it’s also involved in bone growth and maintenance.
Levicept has found a way to modify the NGF pathway without completely removing NGF. They have now finished a small-scale phase I trial mainly designed to test safety rather than efficacy. “We demonstrated that Levi-04 is safe and that it bound to its target, NGF,” says Westbrook. It has not caused RPOA.
Professor Philip Conaghan, director of the Leeds Institute of Rheumatic and Musculoskeletal Medicine, believes that Levi-04 has potential but urges the need for caution. “At this early stage of development, their molecule looks promising for osteoarthritis pain,” he says. “They will have to watch out for RPOA which is a potential problem.”
Westbrook starts phase II trials with 500 patients this summer to check for potential side effects and test the drug’s efficacy.
There is a real push to find an effective alternative to opioids. “We have a lot of work to do,” says Professor Waxman. “But I am confident that we will be able to develop new, much more effective pain therapies.”
Five Memorable Animals Who Expanded the Scientific Frontier
Laika, a gene-edited pig, was named in honor of the first living creature to orbit the earth, a stray dog named Laika.
Untold numbers of animals have contributed to science, in ways big and small. Studying cows and cowpox helped English doctor Edward Jenner create a smallpox vaccine; Ivan Pavlov's experiments on dogs' reactions to external stimuli heavily influenced modern behavioral psychology.
We have these five animals to thank for some of our most important scientific advancements, from space travel to better organ replacement options.
Scientists still work with rats, rabbits, and other mammals to test cosmetics and pharmaceuticals and to conduct infectious disease research. Most of these animals remain nameless and unknown to the public, but over the years, certain individuals have had an outsize effect. We have these five animals to thank for some of our most important scientific advancements, from space travel to better organ replacement options.
1) LAIKA THE DOG
Laika was the first living creature ever to orbit the Earth. In October 1957, the Soviet Sputnik I ship had made history as the first man-made object sent into Earth's orbit; Premier Nikita Khrushchev was keen to gain another Space Race victory by sending up a canine cosmonaut.
Laika ("barker" in Russian), was a stray dog, reportedly a husky-spitz mix, recruited among several other female strays for the trip. Although the scientists put extensive work into preparing Laika and the other canine finalists—evaluating their reactions to air-pressure variations, training them to adapt to pelvic sanitation devices meant to contain waste, and eventually having them live in pressurized capsules for weeks—there was no expectation that the dog would return to Earth, and only one meal's worth of food was sent up with her.
Sputnik II, six times heavier than its predecessor, launched on November 3, 1957. Soviet broadcasts reported that Laika, fitted out with surgically implanted devices to monitor her heart rate, blood pressure, and breathing rates, survived until November 12; the spacecraft stayed in orbit for five more months, burning up when it re-entered the atmosphere.
At the time, the Sputnik II team reassured the world that Laika had died painlessly of oxygen deprivation. It was only decades later, in the 1990s, that Oleg Gazenko—one of the scientists and dog trainers assigned to the mission—revealed that Laika had died 5 to 7 hours after launch from a combination of heat and stress. The capsule had overheated, probably as a result of the rushed preparation; after the fourth orbit, the temperature inside Sputnik was over 90 degrees, and it's doubtful she could have survived much past that. "The more time passes, the more I'm sorry about it. We shouldn't have done it," Gazenko said. "We did not learn enough from the mission to justify the death of the dog."
Yet even the four or five orbits that Laika did complete were enough to spur scientists to press on in the effort to send a human into space.
2) HAM THE CHIMP
Four years after Laika's ill-fated flight, a chimpanzee named Ham entered suborbital flight in the American Project Mercury MR-2 mission on January 31, 1961, becoming the first hominid in space—and unlike Laika, he returned to Earth, alive, after a 16-minute flight.
Even though Ham's flight was not destined for orbit, the spacecraft and booster used on his trip were the same combination intended for the first (human) American's trip later that year. If he came back unharmed, NASA's medical team would be prepared to okay astronaut Alan Shepard's flight.
For approximately 18 months before liftoff, Ham was trained to perform simple tasks, like pushing levers, in response to visual and auditory cues. (If he failed, he received an electric shock; correct performance earned him a treat. Pavlov would have been pleased.)
At 37 pounds, Ham was also the heaviest animal to ever make it to space. His vital signs and movements were monitored from Earth, and after a light electric shock from the ground team reminded him of his tasks, he performed his lever-pushing just a bit slower than he had on Earth, verifying that motion would not be seriously impaired in space.
Less than three months after Ham returned to Earth, on April 12, 1961, Soviet cosmonaut Yuri Gagarin became the first human to complete an orbital flight; Shepard was close behind, successfully crewing the MR-3 mission on May 5. For his part, Ham "retired" to the National Zoo in Washington D.C. for 17 years, before being transferred to the North Carolina Zoological Park; he died of liver failure in 1983 at age 26. His grave is at the International Space Hall of Fame in New Mexico.
3) KOKO THE GORILLA
A western lowland gorilla born at the San Francisco Zoo, Hanabi-ko, or "Koko," became famous in the 1970s for her cognitive and communicative abilities. Psychologist Francine "Penny" Patterson, then a doctoral student at Stanford, chose Koko to work on a language research project, teaching her American Sign Language; by age four, Koko demonstrated the ability both to make up new words and to combine known words to express herself creatively, as opposed to simply mimicking her trainer.
Koko's work with Patterson reflected levels of cognition that were higher than non-human primates had previously been thought to have; by the end of her life, her language skills were roughly equivalent to a young child's, with a vocabulary of around 1,000 signs and the ability to understand 2,000 words of spoken English.
An especially impactful study in 2012 showed that Koko had learned to play the recorder, revealing an ability for voluntary breath control that scientists had previously thought was linked closely to speech and could only be developed by humans. Barbara J. King, a biological anthropologist, suggested that Koko's immersion in a human environment may have helped her develop such a skill, and that it might be misleading to consider similar abilities "innate" or lacking in either humans or non-human primates.
Koko's displays of emotions also fascinated the public, especially those that seemed to closely mirror humans': she cared for pet kittens; appeared on Mr. Rogers' Neighborhood and untied the host's shoes for him; acted playfully with Robin Williams during a visit from him, and later expressed grief when told about the comedian's death. Koko died in her sleep in June 2018, at age 46. Patterson continues to run The Gorilla Foundation, which is dedicated to using inter-species communication to motivate conservation efforts.
4) DOLLY THE SHEEP
Dolly—named after country singer Dolly Parton—was the first mammal ever to be cloned from an adult somatic cell, using the process of nuclear transfer. She was born in 1996 as part of research by scientists Keith Campbell and Ian Wilmut of the University of Edinburgh.
By taking a donor cell from an adult sheep's mammary gland, using it to replace the cell nucleus of an unfertilized, developing egg cell, and then bringing the resultant embryo to term, Campbell and Wilmut proved that even a mature cell (one that had developed to perform mammary gland functions) could revert to an embryonic state and go on to develop into any and all parts of a mammal.
Although cloned livestock are legal in the U.S.—the FDA approved the practice in 2008, after determining that there was no difference between the meat and milk of cattle, pigs, and goats—Dolly has had an even bigger impact on stem cell research. The successful test of nuclear transfer proved that it was possible to change a cell's gene expression by changing its nucleus.
Japanese stem cell biologist Shinya Yamanaka, inspired by the birth of Dolly, won the Nobel Prize in 2012 for his adaptation of the technique. He developed induced pluripotent stem cells (iPS cells) by chemically reverting mature cells back to an embryonic-like blank state that is highly desirable for disease research and treatment. This technique allows researchers to work with such stem cells without the ethically charged complication of having to destroy a human embryo in the process.
5) LAIKA THE PIG
Named in honor of the dog who made it to space, the second science-famous Laika was a genetically engineered pig born in China in 2015 as a result of gene editing carried out by Cambridge, MA startup eGenesis and collaborators.* eGenesis aims to create pigs whose organs—hearts, kidneys, lungs, and more—are safe to transplant into people.
Using animal organs in humans (xenotransplantation) is tricky: the immune system is very good at recognizing interlopers, and the human body can start to reject an organ from another species in as little as five minutes. But pigs are otherwise exceptionally good potential donors for humans: their organs' sizes and functions are very similar, and their quick gestation and maturation make them attractive from an efficiency standpoint, given that twenty Americans die every day waiting for organ donors.
Perhaps unsurprisingly, Dolly the sheep helped move xenotransplantation forward. In the 1990s, immunologist David Sachs was able to use a similar cloning method to eliminate alpha-gal, an enzyme that is produced by most animals with immune systems, including pigs—but not humans. Since our immune systems don't recognize alpha-gal, attacks on that enzyme are a major cause of organ rejection. Sachs' experiments increased the survival time of pig organs in primates to weeks: a huge improvement, but not nearly enough for someone in need of a liver or heart.
The advent of CRISPR technology, and the ability to edit genes, has allowed another leap. In 2015, researchers at eGenesis used targeted gene-editing to eliminate the genes for porcine endogenous retroviruses from pig kidney cells. These viral elements are part of all pigs' genomes and pose a potentially high risk of infecting human cells. (After the HIV/AIDS crisis especially, there was a lot of anxiety about potentially introducing a new virus into the human population.)
The eGenesis lab used nuclear transfer to embed the edited nuclei into egg cells taken from a normal pig; and Laika was born months later—without the dangerous viral genes. eGenesis is now working to make the organs even more humanlike, with the goal of one day providing organs to every human patient in need.
*[Disclosure: In 2019, eGenesis received a series B investment from Leaps By Bayer, the funding sponsor of leapsmag. However, leapsmag is editorially independent of Bayer and is under no obligation to cover companies they invest in.]
[Correction, March 3, 2020: Laika the gene-edited pig was born in China, not Cambridge, and eGenesis is pursuing xenotransplant programs that include heart, kidney, and lung, but not skin, as originally written.]
A Surprising Breakthrough Will Allow Tiny Implants to Fix—and Even Upgrade—Your Body
The medical implants of the future will prompt lively discussion around the boundaries between treatment and enhancement.
Imagine it's the year 2040 and you're due for your regular health checkup. Time to schedule your next colonoscopy, Pap smear if you're a woman, and prostate screen if you're a man.
"The evolution of the biological ion transistor technology is a game changer."
But wait, you no longer need any of those, since you recently got one of the new biomed implants – a device that integrates seamlessly with body tissues, because of a watershed breakthrough that happened in the early 2020s. It's an improved biological transistor driven by electrically charged particles that move in and out of your own cells. Like insulin pumps and cardiac pacemakers, the medical implants of the future will go where they are needed, on or inside the body.
But unlike current implants, biological transistors will have a remarkable range of applications. Currently small enough to fit between a patient's hair follicles, the devices could one day enable correction of problems ranging from damaged heart muscle to failing retinas to deficiencies of hormones and enzymes.
Their usefulness raises the prospect of overcorrection to the point of human enhancement, as in the bionic parts that were imagined on the ABC television series The Six Million Dollar Man, which aired in the 1970s.
"The evolution of the biological ion transistor technology is a game changer," says Zoltan Istvan, who ran as a U.S. Presidential candidate in 2016 for the Transhumanist Party and later ran for California governor. Istvan envisions humans becoming faster, stronger, and increasingly more capable by way of technological innovations, especially in the biotechnology realm. "It's a big step forward on how we can improve and upgrade the human body."
How It Works
The new transistors are more like the soft, organic machines that biology has evolved than like traditional transistors built of semiconductors and metal, according to electric engineering expert Dion Khodagholy, one of the leaders of the team at Columbia University that developed the technology.
The key to the advance, notes Khodagholy, is that the transistors will interface seamlessly with tissue, because the electricity will be of the biological type -- transmitted via the flow of ions through liquid, rather than electrons through metal. This will boost the sensitivity of detection and decoding of biological change.
Naturally, such a paradigm change in the world of medical devices raises potential societal and ethical dilemmas.
Known as an ion-gated transistor (IGT), the new class of technology effectively melds electronics with molecules of human skin. That's the current prototype, but ultimately, biological devices will be able to go anywhere in the body. "IGT-based devices hold great promise for development of fully implantable bioelectronic devices that can address key clinical issues for patients with neuropsychiatric disease," says Khodagholy, based on the expectation that future devices could fuse with, measure, and modulate cells of the human nervous system.
Ethical Implications
Naturally, such a paradigm change in the world of medical devices raises potential societal and ethical dilemmas, starting with who receives the new technology and who pays for it. But, according clinical ethicist and health care attorney David Hoffman, we can gain insight from past experience, such as how society reacted to the invention of kidney dialysis in the mid 20th century.
"Kidney dialysis has been federally funded for all these decades, largely because the who-gets-the-technology question was an issue when the technology entered clinical medicine," says Hoffman, who teaches bioethics at Columbia's College of Physicians and Surgeons as well as at the law school and medical school of Yeshiva University. Just as dialysis became a necessity for many patients, he suggests that the emerging bio-transistors may also become critical life-sustaining devices, prompting discussions about federal coverage.
But unlike dialysis, biological transistors could allow some users to become "better than well," making it more similar to medication for ADHD (attention deficit hyperactivity disorder): People who don't require it can still use it to improve their baseline normal functioning. This raises the classic question: Should society draw a line between treatment and enhancement? And who gets to decide the answer?
If it's strictly a medical use of the technology, should everyone who needs it get to use it, regardless of ability to pay, relying on federal or private insurance coverage? On the other hand, if it's used voluntarily for enhancement, should that option also be available to everyone -- but at an upfront cost?
From a transhumanist viewpoint, getting wrapped up with concerns about the evolution of devices from therapy to enhancement is not worth the trouble.
It seems safe to say that some lively debates and growing pains are on the horizon.
"Even if [the biological ion transistor] is developed only for medical devices that compensate for losses and deficiencies similar to that of a cardiac pacemaker, it will be hard to stop its eventual evolution from compensation to enhancement," says Istvan. "If you use it in a bionic eye to restore vision to the blind, how do you draw the line between replacement of normal function and provision of enhanced function? Do you pass a law placing limits on visual capabilities of a synthetic eye? Transhumanists would oppose such laws, and any restrictions in one country or another would allow another country to gain an advantage by creating their own real-life super human cyborg citizens."
In the same breath though, Istvan admits that biotechnology on a bionic scale is bound to complicate a range of international phenomena, from economic growth and military confrontations to sporting events like the Olympic Games.
The technology is already here, and it's just a matter of time before we see clinically viable, implantable devices. As for how society will react, it seems safe to say that some lively debates and growing pains are on the horizon.






