DNA gathered from animal poop helps protect wildlife

Alida de Flamingh and her team are collecting elephant dung. It holds a trove of information about animal health, diet and genetic diversity.
On the savannah near the Botswana-Zimbabwe border, elephants grazed contentedly. Nearby, postdoctoral researcher Alida de Flamingh watched and waited. As the herd moved away, she went into action, collecting samples of elephant dung that she and other wildlife conservationists would study in the months to come. She pulled on gloves, took a swab, and ran it all over the still-warm, round blob of elephant poop.
Sequencing DNA from fecal matter is a safe, non-invasive way to track and ultimately help protect over 42,000 species currently threatened by extinction. Scientists are using this DNA to gain insights into wildlife health, genetic diversity and even the broader environment. Applied to elephants, chimpanzees, toucans and other species, it helps scientists determine the genetic diversity of groups and linkages with other groups. Such analysis can show changes in rates of inbreeding. Populations with greater genetic diversity adapt better to changes and environmental stressors than those with less diversity, thus reducing their risks of extinction, explains de Flamingh, a postdoctoral researcher at the University of Illinois Urbana-Champaign.
Analyzing fecal DNA also reveals information about an animal’s diet and health, and even nearby flora that is eaten. That information gives scientists broader insights into the ecosystem, and the findings are informing conservation initiatives. Examples include restoring or maintaining genetic connections among groups, ensuring access to certain foraging areas or increasing diversity in captive breeding programs.
Approximately 27 percent of mammals and 28 percent of all assessed species are close to dying out. The IUCN Red List of threatened species, simply called the Red List, is the world’s most comprehensive record of animals’ risk of extinction status. The more information scientists gather, the better their chances of reducing those risks. In Africa, populations of vertebrates declined 69 percent between 1970 and 2022, according to the World Wildlife Fund (WWF).
“We put on sterile gloves and use a sterile swab to collect wet mucus and materials from the outside of the dung ball,” says Alida de Flamingh, a postdoctoral researcher at the University of Illinois Urbana-Champaign.
“When people talk about species, they often talk about ecosystems, but they often overlook genetic diversity,” says Christina Hvilsom, senior geneticist at the Copenhagen Zoo. “It’s easy to count (individuals) to assess whether the population size is increasing or decreasing, but diversity isn’t something we can see with our bare eyes. Yet, it’s actually the foundation for the species and populations.” DNA analysis can provide this critical information.
Assessing elephants’ health
“Africa’s elephant populations are facing unprecedented threats,” says de Flamingh, the postdoc, who has studied them since 2009. Challenges include ivory poaching, habitat destruction and smaller, more fragmented habitats that result in smaller mating pools with less genetic diversity. Additionally, de Flamingh studies the microbial communities living on and in elephants – their microbiomes – looking for parasites or dangerous microbes.
Approximately 415,000 elephants inhabit Africa today, but de Flamingh says the number would be four times higher without these challenges. The IUCN Red List reports African savannah elephants are endangered and African forest elephants are critically endangered. Elephants support ecosystem biodiversity by clearing paths that help other species travel. Their very footprints create small puddles that can host smaller organisms such as tadpoles. Elephants are often described as ecosystems’ engineers, so if they disappear, the rest of the ecosystem will suffer too.
There’s a process to collecting elephant feces. “We put on sterile gloves (which we change for each sample) and use a sterile swab to collect wet mucus and materials from the outside of the dung ball,” says de Flamingh. They rub a sample about the size of a U.S. quarter onto a paper card embedded with DNA preservation technology. Each card is air dried and stored in a packet of desiccant to prevent mold growth. This way, samples can be stored at room temperature indefinitely without the DNA degrading.
Earlier methods required collecting dung in bags, which needed either refrigeration or the addition of preservatives, or the riskier alternative of tranquilizing the animals before approaching them to draw blood samples. The ability to collect and sequence the DNA made things much easier and safer.
“Our research provides a way to assess elephant health without having to physically interact with elephants,” de Flamingh emphasizes. “We also keep track of the GPS coordinates of each sample so that we can create a map of the sampling locations,” she adds. That helps researchers correlate elephants’ health with geographic areas and their conditions.
Although de Flamingh works with elephants in the wild, the contributions of zoos in the United States and collaborations in South Africa (notably the late Professor Rudi van Aarde and the Conservation Ecology Research Unit at the University of Pretoria) were key in studying this method to ensure it worked, she points out.
Protecting chimpanzees
Genetic work with chimpanzees began about a decade ago. Hvilsom and her group at the Copenhagen Zoo analyzed DNA from nearly 1,000 fecal samples collected between 2003 and 2018 by a team of international researchers. The goal was to assess the status of the West African subspecies, which is critically endangered after rapid population declines. Of the four subspecies of chimpanzees, the West African subspecies is considered the most at-risk.
In total, the WWF estimates the numbers of chimpanzees inhabiting Africa’s forests and savannah woodlands at between 173,000 and 300,000. Poaching, disease and human-caused changes to their lands are their major risks.
By analyzing genetics obtained from fecal samples, Hvilsom estimated the chimpanzees’ population, ascertained their family relationships and mapped their migration routes.
“One of the threats is mining near the Nimba Mountains in Guinea,” a stronghold for the West African subspecies, Hvilsom says. The Nimba Mountains are a UNESCO World Heritage Site, but they are rich in iron ore, which is used to make the steel that is vital to the Asian construction boom. As she and colleagues wrote in a recent paper, “Many extractive industries are currently developing projects in chimpanzee habitat.”
Analyzing DNA allows researchers to identify individual chimpanzees more accurately than simply observing them, she says. Normally, field researchers would install cameras and manually inspect each picture to determine how many chimpanzees were in an area. But, Hvilsom says, “That’s very tricky. Chimpanzees move a lot and are fast, so it’s difficult to get clear pictures. Often, they find and destroy the cameras. Also, they live in large areas, so you need a lot of cameras.”
By analyzing genetics obtained from fecal samples, Hvilsom estimated the chimpanzees’ population, ascertained their family relationships and mapped their migration routes based upon DNA comparisons with other chimpanzee groups. The mining companies and builders are using this information to locate future roads where they won’t disrupt migration – a more effective solution than trying to build artificial corridors for wildlife.
“The current route cuts off communities of chimpanzees,” Hvilsom elaborates. That effectively prevents young adult chimps from joining other groups when the time comes, eventually reducing the currently-high levels of genetic diversity.
“The mining company helped pay for the genetics work,” Hvilsom says, “as part of its obligation to assess and monitor biodiversity and the effect of the mining in the area.”
Of 50 toucan subspecies, 11 are threatened or near-threatened with extinction because of deforestation and poaching.
Identifying toucan families
Feces aren't the only substance researchers draw DNA samples from. Jeffrey Coleman, a Ph.D. candidate at the University of Texas at Austin relies on blood tests for studying the genetic diversity of toucans---birds species native to Central America and nearby regions. They live in the jungles, where they hop among branches, snip fruit from trees, toss it in the air and catch it with their large beaks. “Toucans are beautiful, charismatic birds that are really important to the ecosystem,” says Coleman.
Of their 50 subspecies, 11 are threatened or near-threatened with extinction because of deforestation and poaching. “When people see these aesthetically pleasing birds, they’re motivated to care about conservation practices,” he points out.
Coleman works with the Dallas World Aquarium and its partner zoos to analyze DNA from blood draws, using it to identify which toucans are related and how closely. His goal is to use science to improve the genetic diversity among toucan offspring.
Specifically, he’s looking at sections of the genome of captive birds in which the nucleotides repeat multiple times, such as AGATAGATAGAT. Called microsatellites, these consecutively-repeating sections can be passed from parents to children, helping scientists identify parent-child and sibling-sibling relationships. “That allows you to make strategic decisions about how to pair (captive) individuals for mating...to avoid inbreeding,” Coleman says.
Jeffrey Coleman is studying the microsatellites inside the toucan genomes.
Courtesy Jeffrey Coleman
The alternative is to use a type of analysis that looks for a single DNA building block – a nucleotide – that differs in a given sequence. Called single nucleotide polymorphisms (SNPs, pronounced “snips”), they are very common and very accurate. Coleman says they are better than microsatellites for some uses. But scientists have already developed a large body of microsatellite data from multiple species, so microsatellites can shed more insights on relations.
Regardless of whether conservation programs use SNPs or microsatellites to guide captive breeding efforts, the goal is to help them build genetically diverse populations that eventually may supplement endangered populations in the wild. “The hope is that the ecosystem will be stable enough and that the populations (once reintroduced into the wild) will be able to survive and thrive,” says Coleman. History knows some good examples of captive breeding success.
The California condor, which had a total population of 27 in 1987, when the last wild birds were captured, is one of them. A captive breeding program boosted their numbers to 561 by the end of 2022. Of those, 347 of those are in the wild, according to the National Park Service.
Conservationists hope that their work on animals’ genetic diversity will help preserve and restore endangered species in captivity and the wild. DNA analysis is crucial to both types of efforts. The ability to apply genome sequencing to wildlife conservation brings a new level of accuracy that helps protect species and gives fresh insights that observation alone can’t provide.
“A lot of species are threatened,” Coleman says. “I hope this research will be a resource people can use to get more information on longer-term genealogies and different populations.”
Tiny, tough “water bears” may help bring new vaccines and medicines to sub-Saharan Africa
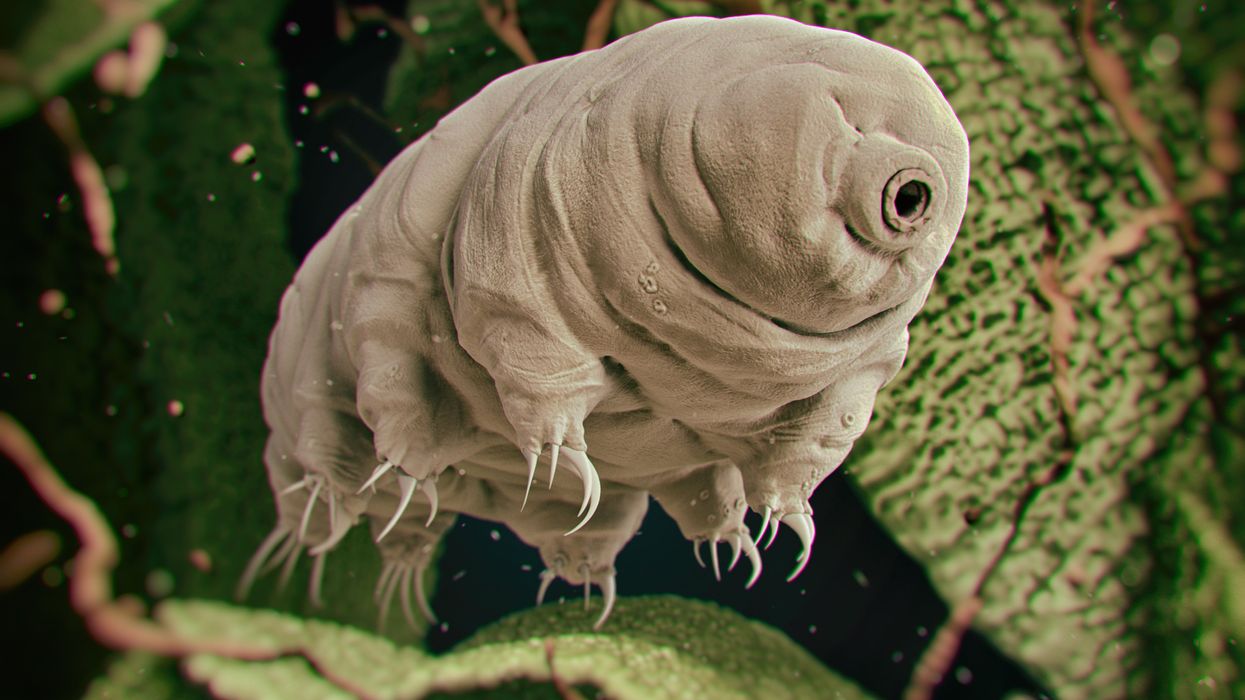
Tardigrades can completely dehydrate and later rehydrate themselves, a survival trick that scientists are harnessing to preserve medicines in hot temperatures.
Microscopic tardigrades, widely considered to be some of the toughest animals on earth, can survive for decades without oxygen or water and are thought to have lived through a crash-landing on the moon. Also known as water bears, they survive by fully dehydrating and later rehydrating themselves – a feat only a few animals can accomplish. Now scientists are harnessing tardigrades’ talents to make medicines that can be dried and stored at ambient temperatures and later rehydrated for use—instead of being kept refrigerated or frozen.
Many biologics—pharmaceutical products made by using living cells or synthesized from biological sources—require refrigeration, which isn’t always available in many remote locales or places with unreliable electricity. These products include mRNA and other vaccines, monoclonal antibodies and immuno-therapies for cancer, rheumatoid arthritis and other conditions. Cooling is also needed for medicines for blood clotting disorders like hemophilia and for trauma patients.
Formulating biologics to withstand drying and hot temperatures has been the holy grail for pharmaceutical researchers for decades. It’s a hard feat to manage. “Biologic pharmaceuticals are highly efficacious, but many are inherently unstable,” says Thomas Boothby, assistant professor of molecular biology at University of Wyoming. Therefore, during storage and shipping, they must be refrigerated at 2 to 8 degrees Celsius (35 to 46 degrees Fahrenheit). Some must be frozen, typically at -20 degrees Celsius, but sometimes as low -90 degrees Celsius as was the case with the Pfizer Covid vaccine.
For Covid, fewer than 73 percent of the global population received even one dose. The need for refrigerated or frozen handling was partially to blame.
The costly cold chain
The logistics network that ensures those temperature requirements are met from production to administration is called the cold chain. This cold chain network is often unreliable or entirely lacking in remote, rural areas in developing nations that have malfunctioning electrical grids. “Almost all routine vaccines require a cold chain,” says Christopher Fox, senior vice president of formulations at the Access to Advanced Health Institute. But when the power goes out, so does refrigeration, putting refrigerated or frozen medical products at risk. Consequently, the mRNA vaccines developed for Covid-19 and other conditions, as well as more traditional vaccines for cholera, tetanus and other diseases, often can’t be delivered to the most remote parts of the world.
To understand the scope of the challenge, consider this: In the U.S., more than 984 million doses of Covid-19 vaccine have been distributed so far. Each one needed refrigeration that, even in the U.S., proved challenging. Now extrapolate to all vaccines and the entire world. For Covid, fewer than 73 percent of the global population received even one dose. The need for refrigerated or frozen handling was partially to blame.
Globally, the cold chain packaging market is valued at over $15 billion and is expected to exceed $60 billion by 2033.
Adobe Stock
Freeze-drying, also called lyophilization, which is common for many vaccines, isn’t always an option. Many freeze-dried vaccines still need refrigeration, and even medicines approved for storage at ambient temperatures break down in the heat of sub-Saharan Africa. “Even in a freeze-dried state, biologics often will undergo partial rehydration and dehydration, which can be extremely damaging,” Boothby explains.
The cold chain is also very expensive to maintain. The global pharmaceutical cold chain packaging market is valued at more than $15 billion, and is expected to exceed $60 billion by 2033, according to a report by Future Market Insights. This cost is only expected to grow. According to the consulting company Accenture, the number of medicines that require the cold chain are expected to grow by 48 percent, compared to only 21 percent for non-cold-chain therapies.
Tardigrades to the rescue
Tardigrades are only about a millimeter long – with four legs and claws, and they lumber around like bears, thus their nickname – but could provide a big solution. “Tardigrades are unique in the animal kingdom, in that they’re able to survive a vast array of environmental insults,” says Boothby, the Wyoming professor. “They can be dried out, frozen, heated past the boiling point of water and irradiated at levels that are thousands of times more than you or I could survive.” So, his team is gradually unlocking tardigrades’ survival secrets and applying them to biologic pharmaceuticals to make them withstand both extreme heat and desiccation without losing efficacy.
Boothby’s team is focusing on blood clotting factor VIII, which, as the name implies, causes blood to clot. Currently, Boothby is concentrating on the so-called cytoplasmic abundant heat soluble (CAHS) protein family, which is found only in tardigrades, protecting them when they dry out. “We showed we can desiccate a biologic (blood clotting factor VIII, a key clotting component) in the presence of tardigrade proteins,” he says—without losing any of its effectiveness.
The researchers mixed the tardigrade protein with the blood clotting factor and then dried and rehydrated that substance six times without damaging the latter. This suggests that biologics protected with tardigrade proteins can withstand real-world fluctuations in humidity.
Furthermore, Boothby’s team found that when the blood clotting factor was dried and stabilized with tardigrade proteins, it retained its efficacy at temperatures as high as 95 degrees Celsius. That’s over 200 degrees Fahrenheit, much hotter than the 58 degrees Celsius that the World Meteorological Organization lists as the hottest recorded air temperature on earth. In contrast, without the protein, the blood clotting factor degraded significantly. The team published their findings in the journal Nature in March.
Although tardigrades rarely live more than 2.5 years, they have survived in a desiccated state for up to two decades, according to Animal Diversity Web. This suggests that tardigrades’ CAHS protein can protect biologic pharmaceuticals nearly indefinitely without refrigeration or freezing, which makes it significantly easier to deliver them in locations where refrigeration is unreliable or doesn’t exist.
The tricks of the tardigrades
Besides the CAHS proteins, tardigrades rely on a type of sugar called trehalose and some other protectants. So, rather than drying up, their cells solidify into rigid, glass-like structures. As that happens, viscosity between cells increases, thereby slowing their biological functions so much that they all but stop.
Now Boothby is combining CAHS D, one of the proteins in the CAHS family, with trehalose. He found that CAHS D and trehalose each protected proteins through repeated drying and rehydrating cycles. They also work synergistically, which means that together they might stabilize biologics under a variety of dry storage conditions.
“We’re finding the protective effect is not just additive but actually is synergistic,” he says. “We’re keen to see if something like that also holds true with different protein combinations.” If so, combinations could possibly protect against a variety of conditions.
Commercialization outlook
Before any stabilization technology for biologics can be commercialized, it first must be approved by the appropriate regulators. In the U.S., that’s the U.S. Food and Drug Administration. Developing a new formulation would require clinical testing and vast numbers of participants. So existing vaccines and biologics likely won’t be re-formulated for dry storage. “Many were developed decades ago,” says Fox. “They‘re not going to be reformulated into thermo-stable vaccines overnight,” if ever, he predicts.
Extending stability outside the cold chain, even for a few days, can have profound health, environmental and economic benefits.
Instead, this technology is most likely to be used for the new products and formulations that are just being created. New and improved vaccines will be the first to benefit. Good candidates include the plethora of mRNA vaccines, as well as biologic pharmaceuticals for neglected diseases that affect parts of the world where reliable cold chain is difficult to maintain, Boothby says. Some examples include new, more effective vaccines for malaria and for pathogenic Escherichia coli, which causes diarrhea.
Tallying up the benefits
Extending stability outside the cold chain, even for a few days, can have profound health, environmental and economic benefits. For instance, MenAfriVac, a meningitis vaccine (without tardigrade proteins) developed for sub-Saharan Africa, can be stored at up to 40 degrees Celsius for four days before administration. “If you have a few days where you don’t need to maintain the cold chain, it’s easier to transport vaccines to remote areas,” Fox says, where refrigeration does not exist or is not reliable.
Better health is an obvious benefit. MenAfriVac reduced suspected meningitis cases by 57 percent in the overall population and more than 99 percent among vaccinated individuals.
Lower healthcare costs are another benefit. One study done in Togo found that the cold chain-related costs increased the per dose vaccine price up to 11-fold. The ability to ship the vaccines using the usual cold chain, but transporting them at ambient temperatures for the final few days cut the cost in half.
There are environmental benefits, too, such as reducing fuel consumption and greenhouse gas emissions. Cold chain transports consume 20 percent more fuel than non-cold chain shipping, due to refrigeration equipment, according to the International Trade Administration.
A study by researchers at Johns Hopkins University compared the greenhouse gas emissions of the new, oral Vaxart COVID-19 vaccine (which doesn’t require refrigeration) with four intramuscular vaccines (which require refrigeration or freezing). While the Vaxart vaccine is still in clinical trials, the study found that “up to 82.25 million kilograms of CO2 could be averted by using oral vaccines in the U.S. alone.” That is akin to taking 17,700 vehicles out of service for one year.
Although tardigrades’ protective proteins won’t be a component of biologic pharmaceutics for several years, scientists are proving that this approach is viable. They are hopeful that a day will come when vaccines and biologics can be delivered anywhere in the world without needing refrigerators or freezers en route.
Jamie Rettinger with his now fiance Amie Purnel-Davis, who helped him through the clinical trial.
Jamie Rettinger was still in his thirties when he first noticed a tiny streak of brown running through the thumbnail of his right hand. It slowly grew wider and the skin underneath began to deteriorate before he went to a local dermatologist in 2013. The doctor thought it was a wart and tried scooping it out, treating the affected area for three years before finally removing the nail bed and sending it off to a pathology lab for analysis.
"I have some bad news for you; what we removed was a five-millimeter melanoma, a cancerous tumor that often spreads," Jamie recalls being told on his return visit. "I'd never heard of cancer coming through a thumbnail," he says. None of his doctors had ever mentioned it either. "I just thought I was being treated for a wart." But nothing was healing and it continued to bleed.
A few months later a surgeon amputated the top half of his thumb. Lymph node biopsy tested negative for spread of the cancer and when the bandages finally came off, Jamie thought his medical issues were resolved.
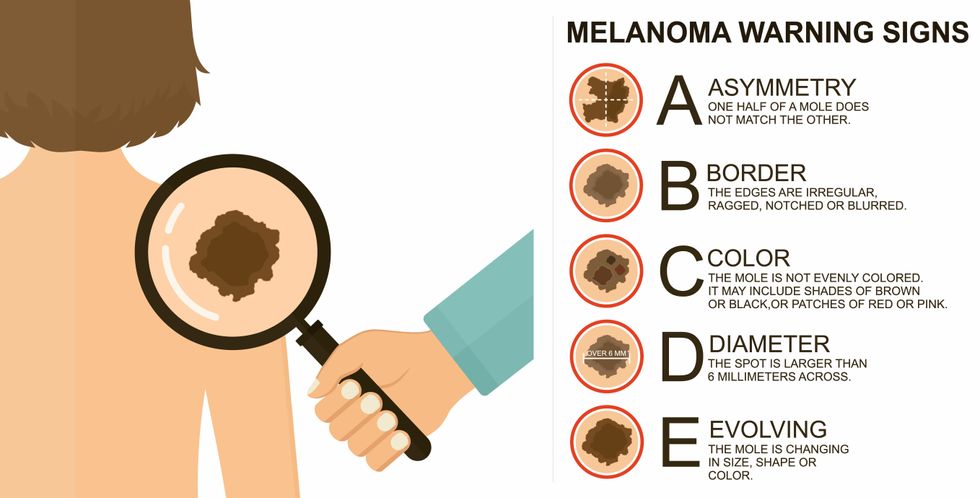
Melanoma is the deadliest form of skin cancer. About 85,000 people are diagnosed with it each year in the U.S. and more than 8,000 die of the cancer when it spreads to other parts of the body, according to the Centers for Disease Control and Prevention (CDC).
There are two peaks in diagnosis of melanoma; one is in younger women ages 30-40 and often is tied to past use of tanning beds; the second is older men 60+ and is related to outdoor activity from farming to sports. Light-skinned people have a twenty-times greater risk of melanoma than do people with dark skin.
"When I graduated from medical school, in 2005, melanoma was a death sentence" --Diwakar Davar.
Jamie had a follow up PET scan about six months after his surgery. A suspicious spot on his lung led to a biopsy that came back positive for melanoma. The cancer had spread. Treatment with a monoclonal antibody (nivolumab/Opdivo®) didn't prove effective and he was referred to the UPMC Hillman Cancer Center in Pittsburgh, a four-hour drive from his home in western Ohio.
An alternative monoclonal antibody treatment brought on such bad side effects, diarrhea as often as 15 times a day, that it took more than a week of hospitalization to stabilize his condition. The only options left were experimental approaches in clinical trials.
Early research
"When I graduated from medical school, in 2005, melanoma was a death sentence" with a cure rate in the single digits, says Diwakar Davar, 39, an oncologist at UPMC Hillman Cancer Center who specializes in skin cancer. That began to change in 2010 with introduction of the first immunotherapies, monoclonal antibodies, to treat cancer. The antibodies attach to PD-1, a receptor on the surface of T cells of the immune system and on cancer cells. Antibody treatment boosted the melanoma cure rate to about 30 percent. The search was on to understand why some people responded to these drugs and others did not.
At the same time, there was a growing understanding of the role that bacteria in the gut, the gut microbiome, plays in helping to train and maintain the function of the body's various immune cells. Perhaps the bacteria also plays a role in shaping the immune response to cancer therapy.
One clue came from genetically identical mice. Animals ordered from different suppliers sometimes responded differently to the experiments being performed. That difference was traced to different compositions of their gut microbiome; transferring the microbiome from one animal to another in a process known as fecal transplant (FMT) could change their responses to disease or treatment.
When researchers looked at humans, they found that the patients who responded well to immunotherapies had a gut microbiome that looked like healthy normal folks, but patients who didn't respond had missing or reduced strains of bacteria.
Davar and his team knew that FMT had a very successful cure rate in treating the gut dysbiosis of Clostridioides difficile, a persistant intestinal infection, and they wondered if a fecal transplant from a patient who had responded well to cancer immunotherapy treatment might improve the cure rate of patients who did not originally respond to immunotherapies for melanoma.
The ABCDE of melanoma detection
Adobe Stock
Clinical trial
"It was pretty weird, I was totally blasted away. Who had thought of this?" Jamie first thought when the hypothesis was explained to him. But Davar's explanation that the procedure might restore some of the beneficial bacterial his gut was lacking, convinced him to try. He quickly signed on in October 2018 to be the first person in the clinical trial.
Fecal donations go through the same safety procedures of screening for and inactivating diseases that are used in processing blood donations to make them safe for transfusion. The procedure itself uses a standard hollow colonoscope designed to screen for colon cancer and remove polyps. The transplant is inserted through the center of the flexible tube.
Most patients are sedated for procedures that use a colonoscope but Jamie doesn't respond to those drugs: "You can't knock me out. I was watching them on the TV going up my own butt. It was kind of unreal at that point," he says. "There were about twelve people in there watching because no one had seen this done before."
A test two weeks after the procedure showed that the FMT had engrafted and the once-missing bacteria were thriving in his gut. More importantly, his body was responding to another monoclonal antibody (pembrolizumab/Keytruda®) and signs of melanoma began to shrink. Every three months he made the four-hour drive from home to Pittsburgh for six rounds of treatment with the antibody drug.
"We were very, very lucky that the first patient had a great response," says Davar. "It allowed us to believe that even though we failed with the next six, we were on the right track. We just needed to tweak the [fecal] cocktail a little better" and enroll patients in the study who had less aggressive tumor growth and were likely to live long enough to complete the extensive rounds of therapy. Six of 15 patients responded positively in the pilot clinical trial that was published in the journal Science.
Davar believes they are beginning to understand the biological mechanisms of why some patients initially do not respond to immunotherapy but later can with a FMT. It is tied to the background level of inflammation produced by the interaction between the microbiome and the immune system. That paper is not yet published.
Surviving cancer
It has been almost a year since the last in his series of cancer treatments and Jamie has no measurable disease. He is cautiously optimistic that his cancer is not simply in remission but is gone for good. "I'm still scared every time I get my scans, because you don't know whether it is going to come back or not. And to realize that it is something that is totally out of my control."
"It was hard for me to regain trust" after being misdiagnosed and mistreated by several doctors he says. But his experience at Hillman helped to restore that trust "because they were interested in me, not just fixing the problem."
He is grateful for the support provided by family and friends over the last eight years. After a pause and a sigh, the ruggedly built 47-year-old says, "If everyone else was dead in my family, I probably wouldn't have been able to do it."
"I never hesitated to ask a question and I never hesitated to get a second opinion." But Jamie acknowledges the experience has made him more aware of the need for regular preventive medical care and a primary care physician. That person might have caught his melanoma at an earlier stage when it was easier to treat.
Davar continues to work on clinical studies to optimize this treatment approach. Perhaps down the road, screening the microbiome will be standard for melanoma and other cancers prior to using immunotherapies, and the FMT will be as simple as swallowing a handful of freeze-dried capsules off the shelf rather than through a colonoscopy. Earlier this year, the Food and Drug Administration approved the first oral fecal microbiota product for C. difficile, hopefully paving the way for more.
An older version of this hit article was first published on May 18, 2021