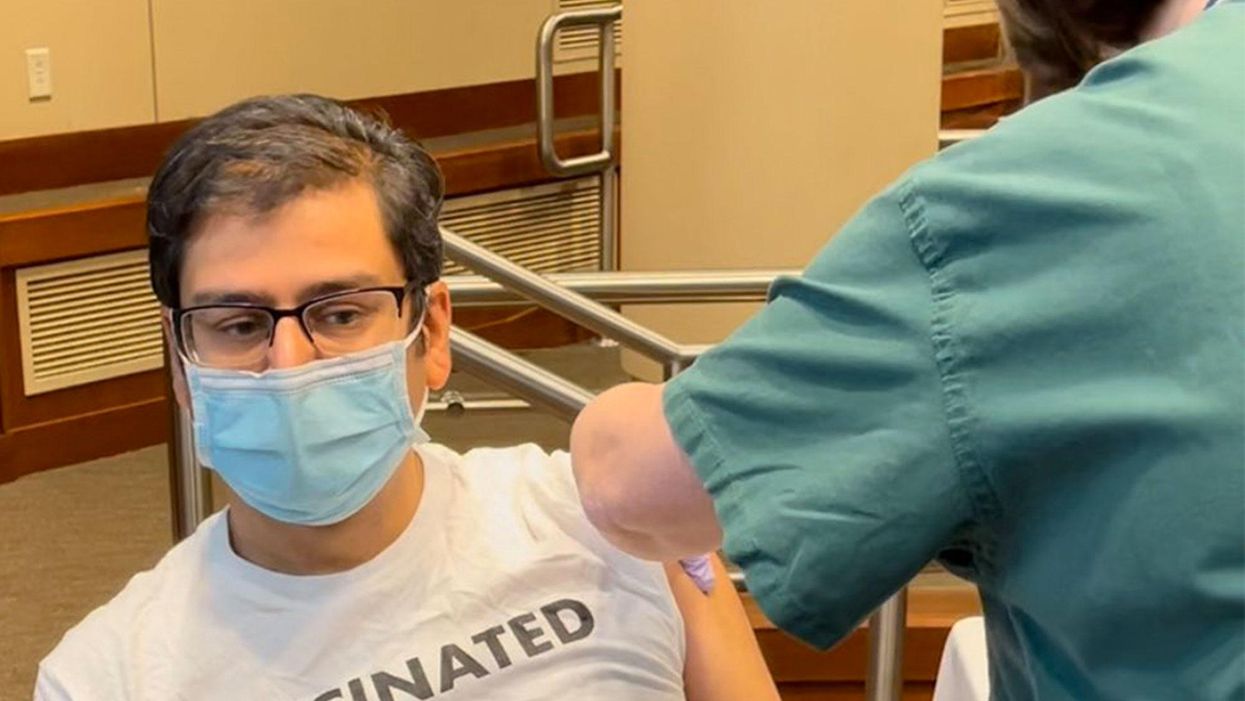
Vaccines Are the Safest Medical Procedure We Have. Make Your Wager Wisely.
Frontline infectious disease physician Amesh Adalja received his COVID-19 vaccine on December 18th, 2020 in Butler, PA.
In the late 1650's the French polymath and renowned scientist Blaise Pascal, having undergone a religious experience that transformed him into something of a zealot, suggested the following logical strategy regarding belief in God: If there is a God, then believing in him will ensure you an eternity of bliss, while not believing in him could earn you an eternal sentence to misery.
On the other hand, if there is no God, believing in him anyway will cost you very little, and not believing in him will mean nothing in the non-existent after life. Therefore, the only sensible bet is to believe in God. This has come to be known as Pascal's wager.
It has a surprising number of applications beyond concerns for a comfortable afterlife. There are many things for which the value of believing something or not can be seen as a cost vs. likely benefit wager, often without regard to the actual truth of the matter. Since science does not profess to have a final truth, and in many areas freely admits its incomplete knowledge, Pascal's wager can provide a useful method of deciding between two alternatives.
For example, it seems that a significant percentage of the population is suspicious of science, or so we are told. We often hear that some large number, approaching or exceeding half of Americans, do not believe in evolution. This seems remarkable on the face of it because there is no viable scientific opposition to evolution and it is widely accepted by biologists and other life-scientists as being fundamental to understanding biology – from genetics to medicine.
What we are not often told is that most of those who answer negatively about believing in evolution nonetheless understand evolution – or at least the basics of it. They are not stupid, ignorant or uninformed. They have simply made a Pascalian wager. What benefit we might ask is derived from believing in evolution rather than a divine creation? Unless you are a professional biologist it is hard to see how this would affect your everyday life. On the other hand professing a belief in Darwinian evolution over the biblical narrative will likely ostracize you from family, friends, co-workers, your church community - in short most of your social infrastructure. Place your bets.
Can we apply any of this to decisions over the current controversy surrounding vaccination – and in particular the newly arrived Covid-19 vaccine?
While it is true that for entirely economic reasons, this is the first vaccine to be produced in this way, the method is not really new and the science that makes it possible has been developing over the last 40 years.
Common Concerns
There are certainly reasons to be concerned about being vaccinated and it would be a gross over-simplification to consider anyone who expresses reticence about taking a vaccine, this new vaccine in particular, as being just plain dumb or scientifically illiterate or gullible. They need be none of these things and still may be suspicious of the vaccine.
One issue is safety. The vaccine, any vaccine, is designed to mobilize your immune system, essentially to fool it into believing that there is an invading virus present and to mount an immune response. That way it will be ready when the real invasion comes, if it comes. This seems pretty sensible and preferable to going to war with an opponent you know nothing about. But still, it is fooling around with Mother Nature and some people are uneasy about that. Although it must be pointed out that the virus is not at all shy about fooling around with your immune system and many other parts of you, so letting it have its way is not good policy either.
What about a vaccine made of genes? This vaccine is being produced by what is being touted as a new method using RNA – genes. While it is true that for entirely economic reasons, this is the first vaccine to be produced in this way, the method is not really new and the science that makes it possible has been developing over the last 40 years. So it's not so radical as the press makes it seem.
But it is true that this method uses RNA, genetic material, to make the vaccine. We hear a lot about gene modification and the potential dangers associated with it. Why then am I going to allow RNA, genes, to be injected into me? The first thing to realize is that this is exactly what the virus does – so whether you get a vaccine or an infection, you are getting genes injected into you. The virus RNA encodes around 12 functional genes (by comparison humans and other mammals have around 25,000 genes). The virus only contains the genes to make a new virus – it does not have any of the capabilities of a normal cell to actually turn those genes into the proteins that make up the complete virus. It hijacks your cells to do this – and that's how it sickens you, by forcing your cells to make new viruses instead of what they should be doing.
Now the new vaccines have taken just one of those genes – the one that directs the production of the now infamous spike protein that appears on the surface of a normal virus – and injects just that one gene into your muscle cells, which then make that one single protein. Your immune system comes along and sees that weird protein and makes antibodies to it. These same antibodies will now recognize the spike protein on the surface of any viral particles that invade your body. We have effectively turned the virus into its own enemy.
The viral RNA that you are getting will decompose over a few days because RNA is not a stable molecule (that, by the way, is why the vaccine needs to be kept frozen) and it will no longer exist in your body. It could only become a permanent part of your genome if it were a DNA molecule instead of an RNA molecule – and even the chances of that happening would be chemically remote. So regardless of how it sounds, this may actually be the safest sort of vaccine to use. In the future it is likely that all vaccines will be made this way.
Then, of course, there is the issue of who is running this whole vaccine program – the government and the pharmaceutical industry. These are the guys who brought you opioid addiction, death by Vioxx, soaring drug prices, the worst health care system in the developed world, regulations where you don't need them and none where you do – am I really going to trust this cast of so-called "inept villains," as some believe, to dictate my personal health choices? Do we know for sure that the claims of efficacy are real or just made up to sell some worthless procedure? It would not be the first time. (I would not, on the other hand, worry about Bill Gates having a chip inserted into you along with the vaccine – if you use any social media, navigational tools, or purchase anything online, then Bill Gates already knows more about you than he will get from any injectable chip. So that train has left the station.)
The main upside to vaccines is that because they use your already existing defense system, they are surprisingly safe.
The Vaccine Wager
All this and a few lesser issues are worth a pause for sure. But we must also look on the positive side of the ledger. Why trust science? Modern medicine and the science behind it has eliminated or dramatically lessened such scourges as smallpox, polio, cholera, chicken pox, measles, rabies and dozens of other killer pathogens that had previously wiped out enormous numbers of people, in some cases significant parts of entire generations. Don't we depend on science for much of the comfort and safety of our everyday lives? Isn't science the way we heat our homes, drive to work, fly around the world, have dependable food? Yes, there is the bomb – but there is also anesthesia.
When it comes to viruses, the only tool we have to fight them is vaccination. The only tool. Antibiotics are for bacteria, a completely different sort of creature. Sanitation beyond personal hand washing is ineffective. Vaccines trick the immune system into recognizing the virus earlier than it would otherwise and protect normal cells from invasion by the virus. Tricking the immune system is understandably problematic for people who believe that their body knows best if it's just kept healthy. This virus, as we have seen from the array of infected people that includes apparently healthy folks, unfortunately does not subscribe to that belief.
By a similar sort of reasoning, some people make the plausible error of calculating that the vaccine is 95% effective but the survival rate is 99%, so why not just let my natural resistance take care of this? Indeed, that might not be unreasonable thinking if we were talking about the common cold, but this virus has shown itself to be a tricky character and we are not yet able to predict who gets a serious case and who a mild one. With those sorts of stakes, you shouldn't wager on either of those numbers because they have nothing to do with you as an individual. Like flipping a coin, there is only a 1% chance of it coming up heads 6 times in a row. But if it has come up heads 5 times in a row the probability of it coming up heads on the next flip is … still 50/50.
An even larger unknown is whether there may be long-term effects associated with SARS-Cov-2, as is the case for many viruses. The 1918 influenza virus has been linked to a subsequent 2-3 fold increase in Parkinson's disease by a mechanism we still don't understand. The virus that gives children chicken pox will hide out in a person's body for 40 years or more and then emerge as a painful, sometimes debilitating, case of shingles. The 99% survivability rate of this virus is meaningless if 20 years from now it causes some devastating pulmonary or brain disease.
The main upside to vaccines is that because they use your already existing defense system, they are surprisingly safe. Safer than antibiotics which have numerous side effects because they are not part of our normal make up and are cell killers – mostly bacterial cells, but they are not so perfectly targeted that they don't leave some collateral damage in their wake. All drugs and treatments have side effects, but vaccines in general have the fewest. This vaccine in particular has undergone many more than the usual safety measures - multiple independent review boards, massive press and public attention, governmental and non-governmental oversight, the most diverse trial cohorts ever assembled. Nothing here was rushed, no shortcuts were taken.
So here's the vaccine wager. Vaccines are the safest medical procedure we have. They are also among the most effective, but that's curiously not important for the bet. My claim about their safety is because vaccines are in a special class of medical tools. They are the only medical procedure or drug that is given to healthy people. Every other treatment we use medically is aimed at some existing pathology - from a cold to cancer.
Vaccines therefore have to reach a higher standard of safety than any other medical treatment. You can't take healthy people and make them sick. Vaccines have fewer side effects than virtually any other drug you wouldn't even think twice about taking – aspirin, for instance, which can cause internal bleeding, gastric ulcers, stroke. But since you are sick when you take those drugs you are willing to make the bet that the benefits will outweigh the possible side effects.
With vaccines the wager is much simpler – it is indeed more like Pascal's original wager. It may or may not be highly effective (some vaccines are only 60% effective) but they are so safe that taking them poses little risk, whereas not taking them subjects you (and others) to considerable risk, i.e., getting the virus. Like believing or not in an afterlife, the smart money is with Pascal, who I think would have reasoned himself right to the head of the vaccination line.
Can Radical Transparency Overcome Resistance to COVID-19 Vaccines?
Secretive panels of independent experts called Data Safety and Monitoring Boards examine clinical trials' data for safety and efficacy.
When historians look back on the COVID-19 pandemic, they may mark November 9, 2020 as the day the tide began to turn. That's when the New York-based pharmaceutical giant Pfizer announced that clinical trials showed its experimental vaccine, developed with the German firm BioNTech, to be 90 percent effective in preventing the disease.
A week later, Massachusetts biotech startup Moderna declared its vaccine to be 95 percent effective. By early December, Great Britain had begun mass inoculations, followed—once the Food and Drug Administration gave the thumbs-up—by the United States. In this scenario, the worst global health crisis in a century was on the cusp of resolution.
Yet future chroniclers may instead peg November 9 as the day false hope dawned. That could happen if serious safety issues, undetected so far, arise after millions of doses are administered. Experts consider it unlikely, however, that such problems alone (as opposed to the panic they might spark) would affect enough people to thwart a victory over the coronavirus. A more immediate obstacle is vaccine hesitancy—the prospect that much of the populace will refuse to roll up their sleeves.
To achieve "herd immunity" for COVID-19 (the point at which a vaccine reduces transmission rates enough to protect those who can't or won't take it, or for whom it doesn't work), epidemiologists estimate that up to 85 percent of the population will have to be vaccinated. Alarmingly, polls suggest that 40 to 50 percent of Americans intend to decline, judging the risks to be more worrisome than those posed by the coronavirus itself.
COVID vaccine skeptics occupy various positions on a spectrum of doubt. Some are committed anti-vaxxers, or devotees of conspiracy theories that view the pandemic as a hoax. Others belong to minority groups that have historically been used as guinea pigs in unethical medical research (for horrific examples, Google "Tuskegee syphilis experiment" or "Henrietta Lacks"). Still others simply mistrust Big Pharma and/or Big Government. A common fear is that the scramble to find a vaccine—intensified by partisan and profit motives—has led to corner-cutting in the testing and approval process. "They really rushed," an Iowa trucker told The Washington Post. "I'll probably wait a couple of months after they start to see how everyone else is handling it."
The COVID crisis has spurred calls for secretive Data Safety and Monitoring Boards to come out of the shadows.
The consensus among scientists, by contrast, is that the process has been rigorous enough, given the exigency of the situation, that the public can feel reasonably confident in any vaccine that has earned the imprimatur of the FDA. For those of us who share that assessment, finding ways to reassure the hesitant-but-persuadable is an urgent matter.
Vax-positive public health messaging is one obvious tactic, but a growing number of experts say it's not enough. They prescribe a regimen of radical transparency throughout the system that regulates research—in particular, regarding the secretive panels that oversee vaccine trials.
The Crucial Role of the Little-Known Panels
Like other large clinical trials involving potentially high-demand or controversial products, studies of COVID-19 vaccines in most countries are supervised by groups of independent observers. Known in the United States as data safety and monitoring boards (DSMBs), and elsewhere as data monitoring committees, these panels consist of scientists, clinicians, statisticians, and other authorities with no ties to the sponsor of the study.
The six trials funded by the federal program known as Operation Warp Speed (including those of newly approved Moderna and frontrunner AstraZeneca) share a DSMB, whose members are selected by the National Institutes of Health; other companies (including Pfizer) appoint their own. The panel's job is to monitor the safety and efficacy of a treatment while the trial is ongoing, and to ensure that data is being collected and analyzed correctly.
Vaccine studies are "double-blinded," which means neither the participants nor the doctors running the trial know who's getting the real thing and who's getting a placebo. But the DSMB can access that information if a study volunteer has what might be a serious side effect—and if the participant was in the vaccine group, the board can ask that the trial be paused for further investigation.
The DSMB also checks for efficacy at pre-determined intervals. If it finds that the vaccine group and the placebo group are getting sick at similar rates, the panel can recommend stopping the trial due to "futility." And if the results look overwhelmingly positive, the DSMB can recommend that the study sponsor apply for FDA approval before the scheduled end of the trial, in order to hurry the product to market.
With this kind of inside dope and high-level influence, DSMBs could easily become targets for outside pressure. That's why, since the 1980s, their membership has typically been kept secret.
During the early days of the AIDS crisis, researchers working on HIV drugs feared for the safety of the experts on their boards. "They didn't want them to be besieged and harassed by members of the community," explains Susan Ellenberg, a professor of biostatistics, medical ethics and health policy at the University of Pennsylvania, and co-author of Data Monitoring Committees in Clinical Trials, the DSMB bible. "You can understand why people would very much want to know how things were looking in a given trial. They wanted to save their own lives; they wanted to save their friends' lives." Ellenberg, who was founding director of the biostatistics branch of the AIDS division at the National Institute of Allergy and Infectious Diseases (NIAID), helped shape a range of policies designed to ensure that DSMBs made decisions based on data and nothing else.
Confidentiality also shields DSMB members from badgering by patient advocacy groups, who might urge that a drug be presented for approval before trial results are conclusive, or by profit-hungry investors. "It prevents people from trying to pry out information to get an edge in the stock market," says Art Caplan, a bioethicist at New York University.
Yet the COVID crisis has spurred calls for DSMBs to come out of the shadows. One triggering event came in March 2020, when the FDA approved hydroxychloroquine for COVID-19—a therapy that President Donald J. Trump touted, despite scant evidence for its efficacy. (Approval was rescinded in June.) If the agency could bow to political pressure on these medications, critics warned, it might do so with vaccines as well. In the end, that didn't happen; the Pfizer approval was issued well after Election Day, despite Trump's goading, and most experts agree that it was based on solid science. Still, public suspicion lingers.
Another shock came in September, after British-based AstraZeneca announced it was pausing its vaccine trial globally due to a "suspected adverse rection" in a volunteer. The company shared no details with the press. Instead, AstraZeneca's CEO divulged them in a private call with J.P. Morgan investors the next day, confirming that the volunteer was suffering from transverse myelitis, a rare and serious spinal inflammation—and that the study had also been halted in July, when another volunteer displayed neurological symptoms. STAT News broke the story after talking to tipsters.
Although both illnesses were found to be unrelated to the vaccine, and the trial was restarted, the incident had a paradoxical effect: while it confirmed for experts that the oversight system was working, AstraZeneca's initial lack of candor added to many laypeople's sense that it wasn't. "If you were seeking to undermine trust, that's kind of how you would go about doing it," says Charles Weijer, a bioethicist at Western University in Ontario, who has helped develop clinical trial guidelines for the World Health Organization.
Both Caplan and Weijer have served on many DSMBs; they believe the boards are generally trustworthy, and that those overseeing COVID vaccine trials are performing their jobs well. But the secrecy surrounding these groups, they and others argue, has become counterproductive. Shining a light on the statistical sausage-makers would help dispel doubts about the finished product.
"I'm not suggesting that any of these companies are doing things unethically," Weijer explains. "But the circumstances of a global pandemic are sufficiently challenging that perhaps they ought to be doing some things differently. I believe it would be trust-producing for data monitoring committees to be more forthcoming than usual."
Building Trust: More Transparency
Just how forthcoming is a matter of debate. Caplan suggests that each COVID vaccine DSMB reveal the name of its chair; that would enable the scientific community, as well as the media and the general public, to get a sense of the integrity and qualifications of the board as a whole while preserving the anonymity of the other members.
Indeed, when Operation Warp Speed's DSMB chair, Richard Whitley, was outed through a website slip-up, many observers applauded his selection for the role; a professor of pediatrics, microbiology, medicine and neurosurgery at the University of Alabama at Birmingham, he is "an exceptionally experienced and qualified individual," Weijer says. (Reporters with ProPublica later identified two other members: Susan Ellenberg and immunologist William Makgoba, known for his work on the South African AIDS Vaccine Initiative.)
Caplan would also like to see more details of the protocols DSMBs are using to make decisions, such as the statistical threshold for efficacy that would lead them to seek approval from the FDA. And he wishes the NIH would spell out specific responsibilities for these monitoring boards. "They don't really have clear, government-mandated charters," he notes. For example, there's no requirement that DSMBs include an ethicist or patient advocate—both of which Caplan considers essential for vaccine trials. "Rough guidelines," he says, "would be useful."
Weijer, for his part, thinks DSMBs should disclose all their members. "When you only disclose the chair, you leave questions unanswered," he says. "What expertise do [the others] bring to the table? Are they similarly free of relevant conflicts of interest? And it doesn't answer the question that will be foremost on many people's minds: are these people in the pocket of pharma?"
Weijer and Caplan both want to see greater transparency around the trial results themselves. Because the FDA approved the Pfizer and Moderna vaccines with emergency use authorizations rather than full licensure, which requires more extensive safety testing, these products reached the market without the usual paper trail of peer-reviewed publications. The same will likely be true of any future COVID vaccines that the agency greenlights. To add another level of scrutiny, both ethicists suggest, each company should publicly release its data at the end of a trial. "That offers the potential for academic groups to go in and do an analysis," Weijer explains, "to verify the claims about the safety and efficacy of the vaccine." The point, he says, is not only to ensure that the approval was justified, but to provide evidence to counter skeptics' qualms.
Caplan may differ on some of the details, but he endorses the premise. "It's all a matter of trust," he says. "You're always watching that, because a vaccine is only as good as the number of people who take it."
Thousands of Vaccine Volunteers Got a Dummy Shot. Should They Get the Real Deal Now?
If a treatment or prevention is known to work, it is considered unethical to withhold it from volunteers in order to prolong a research trial, experts say.
The highly anticipated rollout of a COVID-19 vaccine poses ethical considerations: When will trial volunteers who got a placebo be vaccinated? And how will this affect the data in those trials?
It's an issue that vaccine manufacturers and study investigators are wrestling with as the Food and Drug Administration is expected to grant emergency use authorization this weekend to a vaccine developed by Pfizer and the German company BioNTech. Another vaccine, produced by Moderna, is nearing approval in the United States.
The most vulnerable—health care workers and nursing home residents—are deemed eligible to receive the initial limited supply in accordance with priority recommendations from the Centers for Disease Control and Prevention (CDC).
With health care workers constituting an estimated 20 percent of trial participants, this question also comes to the fore: "Is it now ethically imperative that we offer them the vaccine, those who have had placebo?" says William Schaffner, an infectious diseases physician at Vanderbilt University and an adviser to the CDC's immunization practices committee.
When a "gold-standard" measure becomes available, participants in the placebo group "would ordinarily be notified" of the strong public health recommendation to opt for immunization, says Johan Bester, interim assistant dean for biomedical science education and director of bioethics at the University of Nevada, Las Vegas School of Medicine.
"If a treatment or prevention exists that we know works, it is unethical to withhold it from people who would benefit from it just to answer a research question." This moral principle poses a quandary for ethicists and physicians alike, as they ponder possible paths to proceed with vaccination amid ongoing trials. Rigorous trials are double-blinded—neither the participants nor the investigators know who received the actual vaccine and who got a dummy injection.
"The intent of these trials is to follow these folks for up to two years," says Marci Drees, infection prevention officer and hospital epidemiologist for ChristianaCare in Wilmington, Delaware. At a minimum, she adds, researchers would prefer to monitor participants for six months.
"You can still follow safety over a long-term period of time without actually continuing to have a placebo group for comparison."
But in the midst of a pandemic, that may not be feasible. Prolonged exposure to the highly contagious and lethal virus could have dire consequences.
To avoid compromising the integrity of the blinded data, "there are some potentially creative solutions," Drees says. For instance, trial participants could receive the opposite of what they initially got, whether it was the vaccine or the placebo.
One factor in this decision-making process depends on when a particular trial is slated to conclude. If that time is approaching, the risk of waiting would be lower than if the trial is only halfway in progress, says Eric Lofgren, an epidemiologist at Washington State University who has studied the impact of COVID-19 in jails and at in-person sporting events.
Sometimes a study concludes earlier than the projected completion date. "All clinical trials have a data and safety monitoring board that reviews the interim results," Lofgren says. The board may halt a trial after finding evidence of harm, or when a treatment or vaccine has proven to be "sufficiently good," rendering it unethical to deprive the placebo group of its benefits.
The initial months of a trial are most crucial for assessing a vaccine's safety. Differences between the trial groups would be illuminating if fewer individuals who got the active vaccine contracted the virus and developed symptoms when compared to the placebo recipients. After that point, in vaccine-administered participants, "you can still follow safety over a long-term period of time without actually continuing to have a placebo group for comparison," says Dial Hewlett Jr., medical director for disease control at the Westchester County Department of Health in New York.
Even outside of a trial, safety is paramount and any severe side effects that occur will be closely monitored and investigated through national reporting networks. For example, regulators in the U.K. are investigating several rare but serious allergic reactions to the Pfizer vaccine given on Tuesday. The FDA has asked Pfizer to track allergic reactions in its safety monitoring plan, and some experts are proposing that Pfizer conduct a separate study of the vaccine on people with a history of severe allergies.
As the FDA eventually grants authorization to multiple vaccines, more participants are likely to leave trials and opt to be vaccinated. It is important that enough participants choose to stay in ongoing trials, says Nicole Hassoun, professor of philosophy at the State University of New York at Binghamton, where she directs the Global Health Impact program to extend medical access to the poor.
She's hopeful that younger participants and individuals without underlying medical conditions will make that determination. But the departure of too many participants at high risk for the virus would make it more difficult to evaluate the vaccine's safety and efficacy in those populations, Hassoun says, while acknowledging, "We can't have the best of both worlds."
Once a safe and effective vaccine is approved in the United States, "it would not be ethically appropriate to do placebo trials to test new vaccines."
One solution would entail allowing health care workers to exit a trial after a vaccine is approved, even though this would result in "a conundrum when the next group of people are brought forward to get the vaccine—whether they're people age 65 and older or they're essential workers, or whoever they are," says Vanderbilt physician Schaffner, who is a former board member of the Infectious Diseases Society of America. "All of a sudden, you'll have an erosion of the volunteers who are in the trial."
For now, one way or another, experts agree that current and subsequent trials should proceed. There is a compelling reason to identify additional vaccines with potentially greater effectiveness but with fewer side effects or less complex delivery methods that don't require storage at extremely low temperatures.
"Continuing with existing vaccine trials and starting others remains important," says Nir Eyal, professor and director of Rutgers University's Center for Population-Level Bioethics in New Brunswick, New Jersey. "We still need to tell how much proven vaccines block infections and how long their duration lasts. And populations around the world need vaccines that are easier to store and deliver, or simply cheaper."
But once a safe and effective vaccine is approved in the United States, "it would not be ethically appropriate to do placebo trials to test new vaccines," says bioethicist Bester at the University of Nevada, Las Vegas School of Medicine. "One possibility if a new vaccine emerges, is to test it against existing vaccines."
In a letter sent to trial volunteers in November, Pfizer and BioNTech committed to establishing "a process that would allow interested participants in the placebo group who meet the eligibility criteria for early access in their country to 'cross-over' to the vaccine group." The trial plans to continue monitoring all subjects regardless of whether people in the placebo group cross over, Pfizer said in a presentation to the FDA today. After Pfizer has collected six months of safety data, in April 2021, it plans to ask the FDA for full approval of the vaccine.
In the meantime, the company pledged to update volunteers as they obtain more input from regulatory authorities. "Thank you again for making a difference by being a part of this study," they wrote. "It is only through the efforts of volunteers like you that reaching this important milestone and developing a potential vaccine against COVID-19 is possible."
CORRECTION: An earlier version of this article mistakenly stated that the FDA would be granting emergency "approval" to the Pfizer/BioNTech vaccine, rather than "emergency use authorization." We regret the error.