The Nose Knows: Dogs Are Being Trained to Detect the Coronavirus

Security workers walk with detection dogs in an airport terminal.
Asher is eccentric and inquisitive. He loves an audience, likes keeping busy, and howls to be let through doors. He is a six-year-old working Cocker Spaniel, who, with five other furry colleagues, has now been trained to sniff body odor samples from humans to detect COVID-19 infections.
As the Delta variant and other new versions of the SARS-CoV-2 virus emerge, public health agencies are once again recommending masking while employers contemplate mandatory vaccination. While PCR tests remain the "gold standard" of COVID-19 tests, they can take hours to flag infections. To accelerate the process, scientists are turning to a new testing tool: sniffer dogs.
At the London School of Hygiene and Tropical Medicine (LSHTM), researchers deployed Asher and five other trained dogs to test sock samples from 200 asymptomatic, infected individuals and 200 healthy individuals. In May, they published the findings of the yearlong study in a preprint, concluding that dogs could identify COVID-19 infections with a high degree of accuracy – they could correctly identify a COVID-positive sample up to 94% of the time and a negative sample up to 92% of the time. The paper has yet to be peer-reviewed.
"Dogs can screen lots of people very quickly – 300 people per dog per hour. This means they could be used in places like airports or public venues like stadiums and maybe even workplaces," says James Logan, who heads the Department of Disease Control at LSHTM, adding that canines can also detect variants of SARS-CoV-2. "We included samples from two variants and the dogs could still detect them."
Detection dogs have been one of the most reliable biosensors for identifying the odor of human disease. According to Gemma Butlin, a spokesperson of Medical Detection Dogs, the UK-based charity that trained canines for the LSHTM study, the olfactory capabilities of dogs have been deployed to detect malaria, Parkinson's disease, different types of cancers, as well as pseudomonas, a type of bacteria known to cause infections in blood, lungs, eyes, and other parts of the human body.
COVID-19 has a distinctive smell — a result of chemicals known as volatile organic compounds released by infected body cells, which give off an odor "fingerprint."
"It's estimated that the percentage of a dog's brain devoted to analyzing odors is 40 times larger than that of a human," says Butlin. "Humans have around 5 million scent receptors dedicated to smell. Dogs have 350 million and can detect odors at parts per trillion. To put this into context, a dog can detect a teaspoon of sugar in a million gallons of water: two Olympic-sized pools full."
According to LSHTM scientists, COVID-19 has a distinctive smell — a result of chemicals known as volatile organic compounds released by infected body cells, which give off an odor "fingerprint." Other studies, too, have revealed that the SARS-CoV-2 virus has a distinct olfactory signature, detectable in the urine, saliva, and sweat of infected individuals. Humans can't smell the disease in these fluids, but dogs can.
"Our research shows that the smell associated with COVID-19 is at least partly due to small and volatile chemicals that are produced by the virus growing in the body or the immune response to the virus or both," said Steve Lindsay, a public health entomologist at Durham University, whose team collaborated with LSHTM for the study. He added, "There is also a further possibility that dogs can actually smell the virus, which is incredible given how small viruses are."
In April this year, researchers from the University of Pennsylvania and collaborators published a similar study in the scientific journal PLOS One, revealing that detection dogs could successfully discriminate between urine samples of infected and uninfected individuals. The accuracy rate of canines in this study was 96%. Similarly, last December, French scientists found that dogs were 76-100% effective at identifying individuals with COVID-19 when presented with sweat samples.
Grandjean Dominique, a professor at France's National Veterinary School of Alfort, who led the French study, said that the researchers used two types of dogs — search and rescue dogs, as they can sniff sweat, and explosive detection dogs, because they're often used at airports to find bomb ingredients. Dogs may very well be as good as PCR tests, said Dominique, but the goal, he added, is not to replace these tests with canines.
In France, the government gave the green light to train hundreds of disease detection dogs and deploy them in airports. "They will act as mass pre-test, and only people who are positive will undergo a PCR test to check their level of infection and the kind of variant," says Dominique. He thinks the dogs will be able to decrease the amount of PCR testing and potentially save money.
Since the accuracy rate for bio-detection dogs is fairly high, scientists think they could prove to be a quick diagnosis and mass screening tool, especially at ports, airports, train stations, stadiums, and public gatherings. Countries like Finland, Thailand, UAE, Italy, Chile, India, Australia, Pakistan, Saudi Arabia, Switzerland, and Mexico are already training and deploying canines for COVID-19 detection. The dogs are trained to sniff the area around a person, and if they find the odor of COVID-19 they will sit or stand back from an individual as a signal that they've identified an infection.
While bio-detection dogs seem promising for cheap, large-volume screening, many of the studies that have been performed to date have been small and in controlled environments. The big question is whether this approach work on people in crowded airports, not just samples of shirts and socks in a lab.
"The next step is 'real world' testing where they [canines] are placed in airports to screen people and see how they perform," says Anna Durbin, professor of international health at the John Hopkins Bloomberg School of Public Health. "Testing in real airports with lots of passengers and competing scents will need to be done."
According to Butlin of Medical Detection Dogs, scalability could be a challenge. However, scientists don't intend to have a dog in every waiting room, detecting COVID-19 or other diseases, she said.
"Dogs are the most reliable bio sensors on the planet and they have proven time and time again that they can detect diseases as accurately, if not more so, than current technological diagnostics," said Butlin. "We are learning from them all the time and what their noses know will one day enable the creation an 'E-nose' that does the same job – imagine a day when your mobile phone can tell you that you are unwell."
7 Reasons Why We Should Not Need Boosters for COVID-19
A top infectious disease physician explains why immunity derived from natural infection and the vaccines should be long-lasting.
There are at least 7 reasons why immunity after vaccination or infection with COVID-19 should likely be long-lived. If durable, I do not think boosters will be necessary in the future, despite CEOs of pharmaceutical companies (who stand to profit from boosters) messaging that they may and readying such boosters. To explain these reasons, let's orient ourselves to the main components of the immune system.
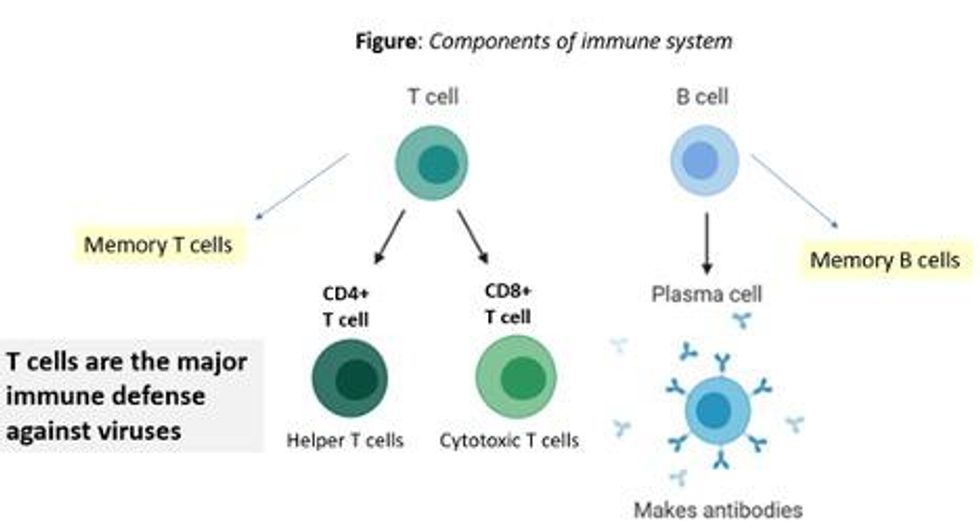
There are two major arms of the immune system: B cells (which produce antibodies) and T cells (which are formed specifically to attack and kill pathogens). T cells are divided into two types, CD4 cells ("helper" T cells) and CD8 cells ("cytotoxic" T cells).
Each arm, once stimulated by infection or vaccine, should hopefully make "memory" banks. So if the body sees the pathogen in the future, these defenses should come roaring back to attack the virus and protect you from getting sick. Plenty of research in COVID-19 indicates a likely long-lasting response to the vaccine or infection. Here are seven of the most compelling reasons:
REASON 1: Memory B Cells Are Produced By Vaccines and Natural Infection
In one study, 12 volunteers who had never had Covid-19--and were fully vaccinated with two Pfizer/BioNTech shots-- underwent biopsies of their lymph nodes. This is where memory B cells are stored in places called "germinal centers". The biopsies were performed three, four, six, and seven weeks after the first mRNA vaccine shot, and were stained to reveal that germinal center memory B cells in the lymph nodes increased in concentration over time.
Natural infection also generates memory B cells. Even after antibody levels wane over time, strong memory B cells were detected in the blood of individuals six and eight months after infection in different studies. Indeed, the half-lives of the memory B cells seen in the study examining patients 8 months after COVID-19 led the authors to conclude that "B cell memory to SARS-CoV-2 was robust and is likely long-lasting." Reason #2 tells us that memory B cells can be active for a very long time indeed.
REASON #2: Memory B Cells Can Produce Neutralizing Antibodies If They See Infection Again Decades Later
Demonstrated production of memory B cells after vaccination or natural infection with COVID-19 is so important because memory B cells, once generated, can be activated to produce high levels of neutralizing antibodies against the pathogen even if encountered many years after the initial exposure. In one amazing study (published in 2008), researchers isolated memory B cells against the 1918 flu strain from the blood of 32 individuals aged 91-101 years. These people had been born on or before 1915 and had survived that pandemic.
Their memory B cells, when exposed to the 1918 flu strain in a test tube, generated high levels of neutralizing antibodies against the virus -- antibodies that then protected mice from lethal infection with this deadly strain. The ability of memory B cells to produce complex antibody responses against an infection nine decades after exposure speaks to their durability.
REASON #3: Vaccines or Natural Infection Trigger Strong Memory T Cell Immunity
All of the trials of the major COVID-19 vaccine candidates measured strong T cell immunity following vaccination, most often assessed by measuring SARS-CoV-2 specific T cells in the phase I/II safety and immunogenicity studies. There are a number of studies that demonstrate the production of strong T cell immunity to COVID-19 after natural infection as well, even when the infection was mild or asymptomatic.
The same study that showed us robust memory B cell production 8 months after natural infection also demonstrated strong and sustained memory T cell production. In fact, the half-lives of the memory T cells in this cohort were long (~125-225 days for CD8+ and ~94-153 days for CD4+ T cells), comparable to the 123-day half-life observed for memory CD8+ T cells after yellow fever immunization (a vaccine usually given once over a lifetime).
A recent study of individuals recovered from COVID-19 show that the initial T cells generated by natural infection mature and differentiate over time into memory T cells that will be "put in the bank" for sustained periods.
REASON #4: T Cell Immunity Following Vaccinations for Other Infections Is Long-Lasting
Last year, we were fortunate to be able to measure how T cell immunity is generated by COVID-19 vaccines, which was not possible in earlier eras when vaccine trials were done for other infections (such as measles, mumps, rubella, pertussis, diphtheria). Antibodies are just the "tip of the iceberg" when assessing the response to vaccination, but were the only arm of the immune response that could be measured following vaccination in the past.
Measuring pathogen-specific T cell responses takes sophisticated technology. However, T cell responses, when assessed years after vaccination for other pathogens, has been shown to be long-lasting. For example, in one study of 56 volunteers who had undergone measles vaccination when they were much younger, strong CD8 and CD4 cell responses to vaccination could be detected up to 34 years later.
REASON #5: T Cell Immunity to Related Coronaviruses That Caused Severe Disease is Long-Lasting
SARS-CoV-2 is a coronavirus that causes severe disease, unlike coronaviruses that cause the common cold. Two other coronaviruses in the recent past caused severe disease, specifically Severely Acute Respiratory Distress Syndrome (SARS) in late 2002-2003 and Middle East Respiratory Syndrome (MERS) in 2011.
A study performed in 2020 demonstrated that the blood of 23 recovered SARS patients possess long-lasting memory T cells that were still reactive to SARS 17 years after the outbreak in 2003. Many scientists expect that T cell immunity to SARS-CoV-2 will be equally durable to that of its cousin.
REASON #6: T Cell Responses from Vaccination and Natural Infection With the Ancestral Strain of COVID-19 Are Robust Against Variants
Even though antibody responses from vaccination may be slightly lower against various COVID-19 variants of concern that have emerged in recent months, T cell immunity after vaccination has been shown to be unperturbed by mutations in the spike protein (in the variants). For instance, T cell responses after mRNA vaccines maintained strong activity against different variants (including P.1 Brazil variant, B.1.1.7 UK variant, B.1.351 South Africa variant and the CA.20.C California variant) in a recent study.
Another study showed that the vaccines generated robust T cell immunity that was unfazed by different variants, including B.1.351 and B.1.1.7. The CD4 and CD8 responses generated after natural infection are equally robust, showing activity against multiple "epitopes" (little segments) of the spike protein of the virus. For instance, CD8 cells responds to 52 epitopes and CD4 cells respond to 57 epitopes across the spike protein, so that a few mutations in the variants cannot knock out such a robust and in-breadth T cell response. Indeed, a recent paper showed that mRNA vaccines were 97.4 percent effective against severe COVID-19 disease in Qatar, even when the majority of circulating virus there was from variants of concern (B.1.351 and B.1.1.7).
REASON #7: Coronaviruses Don't Mutate Quickly Like Influenza, Which Requires Annual Booster Shots
Coronaviruses are RNA viruses, like influenza and HIV (which is actually a retrovirus), but do not mutate as quickly as either one. The reason that coronaviruses don't mutate very rapidly is that their replicating mechanism (polymerase) has a strong proofreading mechanism: If the virus mutates, it usually goes back and self-corrects. Mutations can arise with high rates of replication when transmission is very frequent -- as has been seen in recent months with the emergence of SARS-CoV-2 variants during surges. However, the COVID-19 virus will not be mutating like this when we tamp down transmission with mass vaccination.
In conclusion, I and many of my infectious disease colleagues expect the immunity from natural infection or vaccination to COVID-19 to be durable. Let's put discussion of boosters aside and work hard on global vaccine equity and distribution since the pandemic is not over until it is over for us all.
Professor Tim Caulfield stops by the "Making Sense of Science" podcast this month to discuss the infodemic around COVID-19 and how to deal with it.
The "Making Sense of Science" podcast features interviews with leading medical and scientific experts about the latest developments and the big ethical and societal questions they raise. This monthly podcast is hosted by journalist Kira Peikoff, founding editor of the award-winning science outlet Leaps.org.
Hear the 30-second trailer:
Listen to the whole episode:
.
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.

