Your Questions Answered About Kids, Teens, and Covid Vaccines
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.

On May 13th, scientific and medical experts will discuss and answer questions about the vaccine for those under 16.
This virtual event convened leading scientific and medical experts to address the public's questions and concerns about Covid-19 vaccines in kids and teens. Highlight video below.
DATE:
Thursday, May 13th, 2021
12:30 p.m. - 1:45 p.m. EDT
Dr. H. Dele Davies, M.D., MHCM

Senior Vice Chancellor for Academic Affairs and Dean for Graduate Studies at the University of Nebraska Medical (UNMC). He is an internationally recognized expert in pediatric infectious diseases and a leader in community health.
Dr. Emily Oster, Ph.D.

Professor of Economics at Brown University. She is a best-selling author and parenting guru who has pioneered a method of assessing school safety.
Dr. Tina Q. Tan, M.D.

Professor of Pediatrics at the Feinberg School of Medicine, Northwestern University. She has been involved in several vaccine survey studies that examine the awareness, acceptance, barriers and utilization of recommended preventative vaccines.
Dr. Inci Yildirim, M.D., Ph.D., M.Sc.

Associate Professor of Pediatrics (Infectious Disease); Medical Director, Transplant Infectious Diseases at Yale School of Medicine; Associate Professor of Global Health, Yale Institute for Global Health. She is an investigator for the multi-institutional COVID-19 Prevention Network's (CoVPN) Moderna mRNA-1273 clinical trial for children 6 months to 12 years of age.
About the Event Series
This event is the second of a four-part series co-hosted by Leaps.org, the Aspen Institute Science & Society Program, and the Sabin–Aspen Vaccine Science & Policy Group, with generous support from the Gordon and Betty Moore Foundation and the Howard Hughes Medical Institute.
:
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
The Good, the Bad, and the Ugly in Personalized Medicine
Precision Medicine concept
Is the value of "personalized medicine" over-promised? Why is the quality of health care declining for many people despite the pace of innovation? Do patients and doctors have conflicting priorities? What is the best path forward?
"How do we generate evidence for value, which is what everyone is asking for?"
Some of the country's leading medical experts recently debated these questions at the prestigious annual Personalized Medicine Conference, held at Harvard Medical School in Boston, and LeapsMag was there to bring you the inside scoop.
Personalized Medicine: Is It Living Up to the Hype?
The buzzworthy phrase "personalized medicine" has been touted for years as the way of the future—customizing care to patients based on their predicted responses to treatments given their individual genetic profiles or other analyses. Since the initial sequencing of the human genome around fifteen years ago, the field of genomics has exploded as the costs have dramatically come down – from $2.7 billion to $1000 or less today. Given cheap access to such crucial information, the medical field has been eager to embrace an ultramodern world in which preventing illnesses is status quo, and treatments can be tailored for maximum effectiveness. But whether that world has finally arrived remains debatable.
"I've been portrayed as an advocate for genomics, because I'm excited about it," said Robert C. Green, Director of the Genomes2People Research Program at Harvard Medical School, the Broad Institute, and Brigham and Women's Hospital. He qualified his advocacy by saying that he tries to remain 'equipoised' or balanced in his opinions about the future of personalized medicine, and expressed skepticism about some aspects of its rapid commercialization.
"I have strong feelings about some of the [precision medicine] products that are rushing out to market in both the physician-mediated space and the consumer space," Green said, and challenged the value and sustainability of these products, such as their clinical utility and ability to help produce favorable health outcomes. He asked what most patients and providers want to know, which is, "What are the medical, behavioral, and economic outcomes? How do we generate evidence for value, which is what everyone is asking for?" He later questioned whether the use of 'sexy' and expensive diagnostic technologies is necessarily better than doing things the old-fashioned way. For instance, it is much easier and cheaper to ask a patient directly about their family history of disease, instead of spending thousands of dollars to obtain the same information with pricey diagnostic tests.
"Our mantra is to try to do data-driven health...to catch disease when it occurs early."
Michael Snyder, Professor & Chair of the Department of Genetics and Director of the Center for Genomics and Personalized Medicine at Stanford University, called himself more of an 'enthusiast' about precision medicine products like wearable devices that can digitally track vital signs, including heart rate and blood oxygen levels. "I'm certainly not equipoised," he said, adding, "Our mantra is to try to do data-driven health. We are using this to try to understand health and catch disease when it occurs early."
Snyder then shared his personal account about how his own wearable device alerted him to seek treatment while he was traveling in Norway. "My blood oxygen was low and my heart rate was high, so that told me something was up," he shared. After seeing a doctor, he discovered he was suffering from Lyme disease. He then shared other similar success stories about some of the patients in his department. Using wearable health sensors, he said, could significantly reduce health care costs: "$245 billion is spent every year on diabetes, and if we reduce that by ten percent we just saved $24 billion."

From left, Robert Green, Michael Snyder, Sandro Galea, and Thomas Miller.
(Courtesy Rachele Hendricks-Sturrup)
A Core Reality: Unresolved Societal Issues
Sandro Galea, Dean and Professor at Boston University's School of Public Health, coined himself as a 'skeptic' but also an 'enormous fan' of new technologies. He said, "I want to make sure that you all [the audience] have the best possible treatment for me when I get sick," but added, "In our rush and enthusiasm to embrace personalized and precision medicine approaches, we have done that at the peril of forgetting a lot of core realities."
"There's no one to pay for health care but all of us."
Galea stressed the need to first address certain difficult societal issues because failing to do so will deter precision medicine cures in the future. "Unless we pay attention to domestic violence, housing, racism, poor access to care, and poverty… we are all going to lose," he said. Then he quoted recent statistics about the country's growing gap in both health and wealth, which could potentially erode patient and provider interest in personalized medicine.
Thomas Miller, the founder and partner of a venture capital firm dedicated to advancing precision medicine, agreed with Galea and said that "there's no one to pay for health care but all of us." He recalled witnessing 'abuse' of diagnostic technologies that he had previously invested in. "They were often used as mechanisms to provide unnecessary care rather than appropriate care," he said. "The trend over my 30-year professional career has been that of sensitivity over specificity."
In other words: doctors rely too heavily on diagnostic tools that are sensitive enough to detect signs of a disease, but not accurate enough to confirm the presence of a specific disease. "You will always find that you're sick from something," Miller said. He lamented the counter-productivity and waste brought on by such 'abuse' and added, "That's money that could be used to address some of the problems that you [Galea] just talked about."
Do Patients and Providers Have Conflicting Priorities?
Distrust in the modern health care system is not new in the United States. That fact that medical errors were the third leading cause of death in 2016 may have fueled this mistrust even more. And the level of mistrust appears correlated with race; a recent survey of 118 adults between 18 to 75 years old showed that black respondents were less likely to trust their doctors than the non-Hispanic white respondents. The black respondents were also more concerned about personal privacy and potentially harmful hospital experimentation.
"The vast majority of physicians in this country are incentivized to keep you sick."
As if this context weren't troubling enough, some of the panelists suggested that health care providers and patients have misaligned goals, which may be financially driven.
For instance, Galea stated that health care is currently 'curative' even though that money is better spent on prevention versus cures. "The vast majority of physicians in this country are incentivized to keep you sick," he declared. "They are paid by sick patient visits. Hospital CEOs are paid by the number of sick people they have in their beds." He highlighted this issue as a national priority and mentioned some case studies showing that the behaviors of hospital CEOs quickly change when payment is based on the number of patients in beds versus the number of patients being kept out of the beds. Green lauded Galea's comment as "good sense."
Green also cautioned the audience about potential financial conflicts of interest held by proponents of precision medicine technologies. "Many of the people who are promoting genomics and personalized medicine are people who have financial interests in that arena," he warned. He emphasized that those who are perhaps curbing the over-enthusiasm do not have financial interests at stake.
What is the Best Path Forward for Personalized Medicine?
As useful as personalized medicine may be for selecting the best course of treatment, there is also the flip side: It can allow doctors to predict who will not respond well—and this painful reality must be acknowledged.
Miller argued, "We have a duty to call out therapies that won't work, that will not heal, that need to be avoided, and that will ultimately lead to you saying to a patient, 'There is nothing for you that will work.'"
Although that may sound harsh, it captures the essence of this emerging paradigm, which is to maximize health by using tailored methods that are based on comparative effectiveness, evidence of outcomes, and patient preferences. After all, as Miller pointed out, it wouldn't do much good to prescribe someone a regimen with little reason to think it might help.
For the hype around personalized medicine to be fully realized, Green concluded, "We have to prove to people that [the value of it] is true."
Can Spare Parts from Pigs Solve Our Organ Shortage?
A decellularized small porcine liver.
Jennifer Cisneros was 18 years old, commuting to college from her family's home outside Annapolis, Maryland, when she came down with what she thought was the flu. Over the following weeks, however, her fatigue and nausea worsened, and her weight began to plummet. Alarmed, her mother took her to see a pediatrician. "When I came back with the urine cup, it was orange," Cisneros recalls. "He was like, 'Oh, my God. I've got to send you for blood work.'"
"Eventually, we'll be better off than with a human organ."
Further tests showed that her kidneys were failing, and at Johns Hopkins Hospital, a biopsy revealed the cause: Goodpasture syndrome (GPS), a rare autoimmune disease that attacks the kidneys or lungs. Cisneros was put on dialysis to filter out the waste products that her body could no longer process, and given chemotherapy and steroids to suppress her haywire immune system.
The treatment drove her GPS into remission, but her kidneys were beyond saving. At 19, Cisneros received a transplant, with her mother as donor. Soon, she'd recovered enough to return to school; she did some traveling, and even took up skydiving and parasailing. Then, after less than two years, rejection set in, and the kidney had to be removed.
She went back on dialysis until she was 26, when a stranger learned of her plight and volunteered to donate. That kidney lasted four years, but gave out after a viral infection. Since 2015, Cisneros—now 32, and working as an office administrator between thrice-weekly blood-filtering sessions—has been waiting for a replacement.
She's got plenty of company. About 116,000 people in the United States currently need organ transplants, but fewer than 35,000 organs become available every year. On average, 20 people on the waiting list die each day. And despite repeated campaigns to boost donorship, the gap shows no sign of narrowing.
"This is going to revolutionize medicine, in ways we probably can't yet appreciate."
For decades, doctors and scientists have envisioned a radical solution to the shortage: harvesting other species for spare parts. Xenotransplantation, as the practice is known, could provide an unlimited supply of lifesaving organs for patients like Cisneros. Those organs, moreover, could be altered by genetic engineering or other methods to reduce the danger of rejection—and thus to eliminate the need for immunosuppressive drugs, whose potential side effects include infections, diabetes, and cancer. "Eventually, we'll be better off than with a human organ," says David Cooper, MD, PhD, co-director of the xenotransplant program at the University of Alabama School of Medicine. "This is going to revolutionize medicine, in ways we probably can't yet appreciate."
Recently, progress toward that revolution has accelerated sharply. The cascade of advances began in April 2016, when researchers at the National Heart, Lung, and Blood Institute (NHLBI) reported keeping pig hearts beating in the abdomens of five baboons for a record-breaking mean of 433 days, with one lasting more than two-and-a-half years. Then a team at Emory University announced that a pig kidney sustained a rhesus monkey for 435 days before being rejected, nearly doubling the previous record. At the University of Munich, in Germany, researchers doubled the record for a life-sustaining pig heart transplant in a baboon (replacing the animal's own heart) to 90 days. Investigators at the Salk Institute and the University of California, Davis, declared that they'd grown tissue in pig embryos using human stem cells—a first step toward cultivating personalized replacement organs. The list goes on.
Such breakthroughs, along with a surge of cash from biotech investors, have propelled a wave of bullish media coverage. Yet this isn't the first time that xenotransplantation has been touted as the next big thing. Twenty years ago, the field seemed poised to overcome its final hurdles, only to encounter a setback from which it is just now recovering.
Which raises a question: Is the current excitement justified? Or is the hype again outrunning the science?
A History of Setbacks
The idea behind xenotransplantation dates back at least as far as the 17th century, when French physician Jean-Baptiste Denys tapped the veins of sheep and cows to perform the first documented human blood transfusions. (The practice was banned after two of the four patients died, probably from an immune reaction.) In the 19th century, surgeons began transplanting corneas from pigs and other animals into humans, and using skin xenografts to aid in wound healing; despite claims of miraculous cures, medical historians believe those efforts were mostly futile. In the 1920s and '30s, thousands of men sought renewed vigor through testicular implants from monkeys or goats, but the fad collapsed after studies showed the effects to be imaginary.
Research shut down when scientists discovered a virus in pig DNA that could infect human cells.
After the first successful human organ transplant in 1954—of a kidney, passed between identical twin sisters—the limited supply of donor organs brought a resurgence of interest in animal sources. Attention focused on nonhuman primates, our species' closest evolutionary relatives. At Tulane University, surgeon Keith Reemstma performed the first chimpanzee-to-human kidney transplants in 1963 and '64. Although one of the 13 patients lived for nine months, the rest died within a few weeks due to organ rejection or infections. Other surgeons attempted liver and heart xenotransplants, with similar results. Even the advent of the first immunosuppressant drug, cyclosporine, in 1983, did little to improve survival rates.
In the 1980s, Cooper—a pioneering heart transplant surgeon who'd embraced the dream of xenotransplantation—began arguing that apes and monkeys might not be the best donor animals after all. "First of all, there's not enough of them," he explains. "They breed in ones and twos, and take years to grow to full size. Even then, their hearts aren't big enough for a 70-kg. patient." Pigs, he suggested, would be a more practical alternative. But when he tried transplanting pig organs into nonhuman primates (as surrogates for human recipients), they were rejected within minutes.
In 1992, Cooper's team identified a sugar on the surface of porcine cells, called alpha-1,3-galactose (a-gal), as the main target for the immune system's attack. By then, the first genetically modified pigs had appeared, and biotech companies—led by the Swiss-based pharma giant Novartis—began pouring millions of dollars into developing one whose organs could elude or resist the human body's defenses.
Disaster struck five years later, when scientists reported that a virus whose genetic code was written into pig DNA could infect human cells in lab experiments. Although there was no evidence that the virus, known as PERV (for porcine endogenous retrovirus) could cause disease in people, the discovery stirred fears that xenotransplants might unleash a deadly epidemic. Facing scrutiny from government regulators and protests from anti-GMO and animal-rights activists, Novartis "pulled out completely," Cooper recalls. "They slaughtered all their pigs and closed down their research facility." Competitors soon followed suit.
The riddles surrounding animal-to-human transplants are far from fully solved.
A New Chapter – With New Questions
Yet xenotransplantation's visionaries labored on, aided by advances in genetic engineering and immunosuppression, as well as in the scientific understanding of rejection. In 2003, a team led by Cooper's longtime colleague David Sachs, at Harvard Medical School, developed a pig lacking the gene for a-gal; over the next few years, other scientists knocked out genes expressing two more problematic sugars. In 2013, Muhammad Mohiuddin, then chief of the transplantation section at the NHLBI, further modified a group of triple-knockout pigs, adding genes that code for two human proteins: one that shields cells from attack by an immune mechanism known as the complement system; another that prevents harmful coagulation. (It was those pigs whose hearts recently broke survival records when transplanted into baboon bellies. Mohiuddin has since become director of xenoheart transplantation at the University of Maryland's new Center for Cardiac Xenotransplantation Research.) And in August 2017, researchers at Harvard Medical School, led by George Church and Luhan Yang, announced that they'd used CRISPR-cas9—an ultra-efficient new gene-editing technique—to disable 62 PERV genes in fetal pig cells, from which they then created cloned embryos. Of the 37 piglets born from this experiment, none showed any trace of the virus.
Still, the riddles surrounding animal-to-human transplants are far from fully solved. One open question is what further genetic manipulations will be necessary to eliminate all rejection. "No one is so naïve as to think, 'Oh, we know all the genes—let's put them in and we are done,'" biologist Sean Stevens, another leading researcher, told the The New York Times. "It's an iterative process, and no one that I know can say whether we will do two, or five, or 100 iterations." Adding traits can be dangerous as well; pigs engineered to express multiple anticoagulation proteins, for example, often die of bleeding disorders. "We're still finding out how many you can do, and what levels are acceptable," says Cooper.
Another question is whether PERV really needs to be disabled. Cooper and some of his colleagues note that pig tissue has long been used for various purposes, such as artificial heart valves and wound-repair products, without incident; requiring the virus to be eliminated, they argue, will unnecessarily slow progress toward creating viable xenotransplant organs and the animals that can provide them. Others disagree. "You cannot do anything with pig organs if you do not remove them," insists bioethicist Jeantine Lunshof, who works with Church and Yang at Harvard. "The risk is simply too big."
"We've removed the cells, so we don't have to worry about latent viruses."
Meanwhile, over the past decade, other approaches to xenotransplantation have emerged. One is interspecies blastocyst complementation, which could produce organs genetically identical to the recipient's tissues. In this method, genes that produce a particular organ are knocked out in the donor animal's embryo. The embryo is then injected with pluripotent stem cells made from the tissue of the intended recipient. The stem cells move in to fill the void, creating a functioning organ. This technique has been used to create mouse pancreases in rats, which were then successfully transplanted into mice. But the human-pig "chimeras" recently created by scientists were destroyed after 28 days, and no one plans to bring such an embryo to term anytime soon. "The problem is that cells don't stay put; they move around," explains Father Kevin FitzGerald, a bioethicist at Georgetown University. "If human cells wind up in a pig's brain, that leads to a really interesting conundrum. What if it's self-aware? Are you going to kill it?"
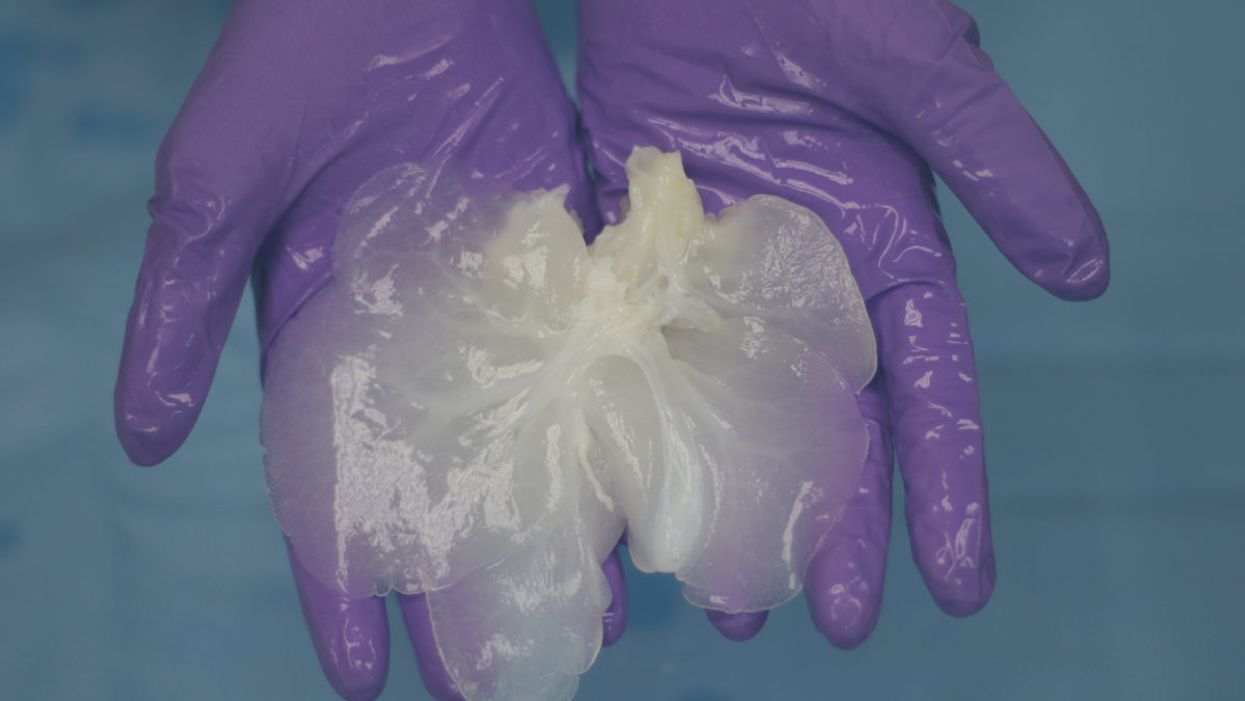
Much further along, and less ethically fraught, is a technique in which decellularized pig organs act as a scaffold for human cells. A Minnesota-based company called Miromatrix Medical is working with Mayo Clinic researchers to develop this method. First, a mild detergent is pumped through the organ, washing away all cellular material. The remaining structure, composed mainly of collagen, is placed in a bioreactor, where it's seeded with human cells. In theory, each type of cell that normally populates the organ will migrate to its proper place (a process that naturally occurs during fetal development, though it remains poorly understood). One potential advantage of this system is that it doesn't require genetically modified pigs; nor will the animals have to be raised under controlled conditions to avoid exposure to transmissible pathogens. Instead, the organs can be collected from ordinary slaughterhouses.

Recellularized livers in bioreactors
(Courtesy of Miromatrix)
"We've removed the cells, so we don't have to worry about latent viruses," explains CEO Jeff Ross, who describes his future product as a bioengineered human organ rather than a xeno-organ. That makes PERV a nonissue. To shorten the pathway to approval by the Food and Drug Administration, the replacement cells will initially come from human organs not suitable for transplant. But eventually, they'll be taken from the recipient (as in blastocyst complementation), which should eliminate the need for immunosuppression.
Clinical trials in xenotransplantation may begin as early as 2020.
Miromatrix plans to offer livers first, followed by kidneys, hearts, and eventually lungs and pancreases. The company recently succeeded in seeding several decellularized pig livers with human and porcine endothelial cells, which flocked obediently to the blood vessels. Transplanted into young pigs, the organs showed unimpaired circulation, with no sign of clotting. The next step is to feed all four liver cell types back into decellularized livers, and see if the transplanted organs will keep recipient pigs alive.
Ross hopes to launch clinical trials by 2020, and several other groups (including Cooper's, which plans to start with kidneys) envision a similar timeline. Investors seem to share their confidence. The biggest backer of xenotransplantation efforts is United Therapeutics, whose founder and co-CEO, Martine Rothblatt, has a daughter with a lung condition that may someday require a transplant; since 2011, the biotech firm has poured at least $100 million into companies pursuing such technologies, while supporting research by Cooper, Mohiuddin, and other leaders in the field. Church and Yang, at Harvard, have formed their own company, eGenesis, bringing in a reported $40 million in funding; Miromatrix has raised a comparable amount.
It's impossible to predict who will win the xenotransplantation race, or whether some new obstacle will stop the competition in its tracks. But Jennifer Cisneros is rooting for all the contestants. "These technologies could save my life," she says. If she hasn't found another kidney before trials begin, she has just one request: "Sign me up."