COVID Vaccines Put Anti-Science Activists to Shame

On a rain-soaked day, thousands marched on Washington, D.C. to fight for science funding and scientific analysis in politics.
It turns out that, despite the destruction and heartbreak caused by the COVID pandemic, there is a silver lining: Scientists from academia, government, and industry worked together and, using the tools of biotechnology, created multiple vaccines that surely will put an end to the worst of the pandemic sometime in 2021. In short, they proved that science works, particularly that which comes from industry. Though politicians and the public love to hate Big Ag and Big Pharma, everybody comes begging for help when the going gets tough.
The change in public attitude is tangible. A headline in the Financial Times declared, "Covid vaccines offer Big Pharma a chance of rehabilitation." In its analysis, the FT says that the pharmaceutical industry is widely reviled because of the high prices it charges for its drugs, among other things, but the speed with which the industry developed COVID vaccines may allow for its reputation to be refurbished.
The Media's Role in Promoting Anti-Biotech Activism
Of course, the media is partly to blame for the pharmaceutical industry's dismal reputation in the first place because of journalists' penchant for oversimplifying complicated stories and pinning blame on an easy scapegoat. While the pharmaceutical industry is far from angelic and places a hefty price tag on its products in the U.S., often gone unmentioned is the fact that high drug prices are the result of multiple factors, including lack of competition (even among generic drugs), foreign price controls that allow citizens of other countries to "free load" off of American consumers, and a deliberately opaque drug supply chain (that involves not only profit-maximizing pharmaceutical manufacturers but "middlemen" like distributors). But why delve into such nuance when it's easier to point to villains like Martin Shkreli?
Big Ag has been subjected to identical mistreatment by the media, with outlets such as the New York Times among the biggest offenders. One article it published compared pesticides to "Nazi-made sarin gas," and another spread misinformation about a high-profile biotech scientist. The website Undark, whose stated mission is "true journalistic coverage of the sciences," once published an opinion piece written by a person who works for an anti-GMO organization and another criticizing Monsanto for its reasonable efforts to defend itself from disinformation. These aren't cherry-picked examples. Overall, the media clearly has taken sides: Science is great, unless it's science from industry.
If the scientific community can use the powerful techniques of biotechnology to cure a previously unknown infectious disease in less than a year, then why shouldn't it be able to cure genetic diseases in humans?
Now, the very same media – which has portrayed the pharmaceutical and biotech industries in the worst possible light, often for political or ideological reasons – is wondering why so many Americans are reluctant to get a COVID vaccine. Perhaps their reportage has something to do with it.
Tech Strikes Back
For years, the agricultural, pharmaceutical, and biotech industries fought back, but to no avail. GMOs are feared, pharma is hated, and biotech is misunderstood. Regulatory red tape abounds. But that may be all about to change, not because of a clever PR campaign, but thanks to the successful coronavirus vaccines produced by the pharma/biotech industry.
All of the major vaccines were created using biotechnology, broadly defined as the use of living systems and organisms to develop products intended to improve human life or the planet. The Pfizer/BioNTech and Moderna vaccines rely on mRNA (messenger RNA), which is essentially a molecular "photocopy" of the more familiar genetic material DNA. The mRNA molecules were tweaked using biotech and then shown to be 95% effective at preventing COVID in human volunteers. The AstraZeneca/Oxford vaccine is based on an older technology that genetically modifies a harmless virus to resemble an immunological target, in this case, SARS-CoV-2. Their vaccine is 62% to 90% effective.
Even better, the pharma/biotech industry showed that it can work hand-in-hand with the government, for instance the FDA, to produce vaccines in record-breaking time. Operation Warp Speed provided some financing to facilitate this process. History will look back at this endeavor and likely conclude that the unprecedented level of cooperation to develop a vaccine in less than 12 months was one of the greatest triumphs in public health history. (The bungled slow rollout is another story.)
Perhaps the most important lesson that society will learn is that the scientific method works.
The pharma/biotech industry has thus gained tremendous momentum. For the first time it seems, those who are opposed to scientific progress and biotechnology are on the defensive. If the scientific community can use the powerful techniques of biotechnology to cure a previously unknown infectious disease in less than a year, then why shouldn't it be able to cure genetic diseases in humans? Or create genetically modified crops that are resistant to insects and drought? Or use genetically modified mosquitoes to help fight against killer diseases like malaria? The arguments against biotechnology have been made exponentially weaker by the success of the coronavirus vaccine.
Perhaps the most important lesson that society will learn is that the scientific method works. We observed (by collecting samples of an unknown virus and sequencing its genome), hypothesized (by predicting which parts of the virus would trigger an immune response), experimented (by recruiting tens of thousands of volunteers into clinical trials), and concluded (that the vaccines worked). It was a thing of pure beauty.
Thanks to all the players involved – from Big Government to Big Pharma – we are beginning the process of being rescued from a modern-day plague. Let us hope that this scientific success also deals a fatal blow to the forces of ignorance that have held back technological progress for decades.
[Editor's Note: LeapsMag is an editorially independent publication that receives program support from Leaps by Bayer. LeapsMag's founding in 2017 predates Bayer's acquisition of Monsanto in 2018. All content published on LeapsMag is strictly free of influence, censorship, and oversight from its corporate sponsor. Read more about LeapsMag's organizational independence here.]
DNA- and RNA-based electronic implants may revolutionize healthcare
The test tubes contain tiny DNA/enzyme-based circuits, which comprise TRUMPET, a new type of electronic device, smaller than a cell.
Implantable electronic devices can significantly improve patients’ quality of life. A pacemaker can encourage the heart to beat more regularly. A neural implant, usually placed at the back of the skull, can help brain function and encourage higher neural activity. Current research on neural implants finds them helpful to patients with Parkinson’s disease, vision loss, hearing loss, and other nerve damage problems. Several of these implants, such as Elon Musk’s Neuralink, have already been approved by the FDA for human use.
Yet, pacemakers, neural implants, and other such electronic devices are not without problems. They require constant electricity, limited through batteries that need replacements. They also cause scarring. “The problem with doing this with electronics is that scar tissue forms,” explains Kate Adamala, an assistant professor of cell biology at the University of Minnesota Twin Cities. “Anytime you have something hard interacting with something soft [like muscle, skin, or tissue], the soft thing will scar. That's why there are no long-term neural implants right now.” To overcome these challenges, scientists are turning to biocomputing processes that use organic materials like DNA and RNA. Other promised benefits include “diagnostics and possibly therapeutic action, operating as nanorobots in living organisms,” writes Evgeny Katz, a professor of bioelectronics at Clarkson University, in his book DNA- And RNA-Based Computing Systems.
While a computer gives these inputs in binary code or "bits," such as a 0 or 1, biocomputing uses DNA strands as inputs, whether double or single-stranded, and often uses fluorescent RNA as an output.
Adamala’s research focuses on developing such biocomputing systems using DNA, RNA, proteins, and lipids. Using these molecules in the biocomputing systems allows the latter to be biocompatible with the human body, resulting in a natural healing process. In a recent Nature Communications study, Adamala and her team created a new biocomputing platform called TRUMPET (Transcriptional RNA Universal Multi-Purpose GatE PlaTform) which acts like a DNA-powered computer chip. “These biological systems can heal if you design them correctly,” adds Adamala. “So you can imagine a computer that will eventually heal itself.”
The basics of biocomputing
Biocomputing and regular computing have many similarities. Like regular computing, biocomputing works by running information through a series of gates, usually logic gates. A logic gate works as a fork in the road for an electronic circuit. The input will travel one way or another, giving two different outputs. An example logic gate is the AND gate, which has two inputs (A and B) and two different results. If both A and B are 1, the AND gate output will be 1. If only A is 1 and B is 0, the output will be 0 and vice versa. If both A and B are 0, the result will be 0. While a computer gives these inputs in binary code or "bits," such as a 0 or 1, biocomputing uses DNA strands as inputs, whether double or single-stranded, and often uses fluorescent RNA as an output. In this case, the DNA enters the logic gate as a single or double strand.
If the DNA is double-stranded, the system “digests” the DNA or destroys it, which results in non-fluorescence or “0” output. Conversely, if the DNA is single-stranded, it won’t be digested and instead will be copied by several enzymes in the biocomputing system, resulting in fluorescent RNA or a “1” output. And the output for this type of binary system can be expanded beyond fluorescence or not. For example, a “1” output might be the production of the enzyme insulin, while a “0” may be that no insulin is produced. “This kind of synergy between biology and computation is the essence of biocomputing,” says Stephanie Forrest, a professor and the director of the Biodesign Center for Biocomputing, Security and Society at Arizona State University.
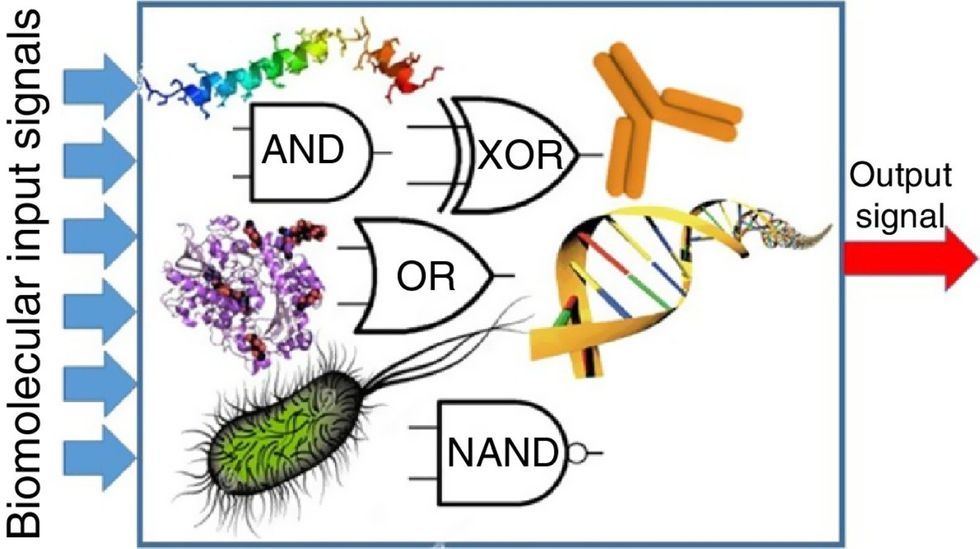
Biocomputing circles are made of DNA, RNA, proteins and even bacteria.
Evgeny Katz
The TRUMPET’s promise
Depending on whether the biocomputing system is placed directly inside a cell within the human body, or run in a test-tube, different environmental factors play a role. When an output is produced inside a cell, the cell's natural processes can amplify this output (for example, a specific protein or DNA strand), creating a solid signal. However, these cells can also be very leaky. “You want the cells to do the thing you ask them to do before they finish whatever their businesses, which is to grow, replicate, metabolize,” Adamala explains. “However, often the gate may be triggered without the right inputs, creating a false positive signal. So that's why natural logic gates are often leaky." While biocomputing outside a cell in a test tube can allow for tighter control over the logic gates, the outputs or signals cannot be amplified by a cell and are less potent.
TRUMPET, which is smaller than a cell, taps into both cellular and non-cellular biocomputing benefits. “At its core, it is a nonliving logic gate system,” Adamala states, “It's a DNA-based logic gate system. But because we use enzymes, and the readout is enzymatic [where an enzyme replicates the fluorescent RNA], we end up with signal amplification." This readout means that the output from the TRUMPET system, a fluorescent RNA strand, can be replicated by nearby enzymes in the platform, making the light signal stronger. "So it combines the best of both worlds,” Adamala adds.
These organic-based systems could detect cancer cells or low insulin levels inside a patient’s body.
The TRUMPET biocomputing process is relatively straightforward. “If the DNA [input] shows up as single-stranded, it will not be digested [by the logic gate], and you get this nice fluorescent output as the RNA is made from the single-stranded DNA, and that's a 1,” Adamala explains. "And if the DNA input is double-stranded, it gets digested by the enzymes in the logic gate, and there is no RNA created from the DNA, so there is no fluorescence, and the output is 0." On the story's leading image above, if the tube is "lit" with a purple color, that is a binary 1 signal for computing. If it's "off" it is a 0.
While still in research, TRUMPET and other biocomputing systems promise significant benefits to personalized healthcare and medicine. These organic-based systems could detect cancer cells or low insulin levels inside a patient’s body. The study’s lead author and graduate student Judee Sharon is already beginning to research TRUMPET's ability for earlier cancer diagnoses. Because the inputs for TRUMPET are single or double-stranded DNA, any mutated or cancerous DNA could theoretically be detected from the platform through the biocomputing process. Theoretically, devices like TRUMPET could be used to detect cancer and other diseases earlier.
Adamala sees TRUMPET not only as a detection system but also as a potential cancer drug delivery system. “Ideally, you would like the drug only to turn on when it senses the presence of a cancer cell. And that's how we use the logic gates, which work in response to inputs like cancerous DNA. Then the output can be the production of a small molecule or the release of a small molecule that can then go and kill what needs killing, in this case, a cancer cell. So we would like to develop applications that use this technology to control the logic gate response of a drug’s delivery to a cell.”
Although platforms like TRUMPET are making progress, a lot more work must be done before they can be used commercially. “The process of translating mechanisms and architecture from biology to computing and vice versa is still an art rather than a science,” says Forrest. “It requires deep computer science and biology knowledge,” she adds. “Some people have compared interdisciplinary science to fusion restaurants—not all combinations are successful, but when they are, the results are remarkable.”
Crickets are low on fat, high on protein, and can be farmed sustainably. They are also crunchy.
In today’s podcast episode, Leaps.org Deputy Editor Lina Zeldovich speaks about the health and ecological benefits of farming crickets for human consumption with Bicky Nguyen, who joins Lina from Vietnam. Bicky and her business partner Nam Dang operate an insect farm named CricketOne. Motivated by the idea of sustainable and healthy protein production, they started their unconventional endeavor a few years ago, despite numerous naysayers who didn’t believe that humans would ever consider munching on bugs.
Yet, making creepy crawlers part of our diet offers many health and planetary advantages. Food production needs to match the rise in global population, estimated to reach 10 billion by 2050. One challenge is that some of our current practices are inefficient, polluting and wasteful. According to nonprofit EarthSave.org, it takes 2,500 gallons of water, 12 pounds of grain, 35 pounds of topsoil and the energy equivalent of one gallon of gasoline to produce one pound of feedlot beef, although exact statistics vary between sources.
Meanwhile, insects are easy to grow, high on protein and low on fat. When roasted with salt, they make crunchy snacks. When chopped up, they transform into delicious pâtes, says Bicky, who invents her own cricket recipes and serves them at industry and public events. Maybe that’s why some research predicts that edible insects market may grow to almost $10 billion by 2030. Tune in for a delectable chat on this alternative and sustainable protein.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
Further reading:
More info on Bicky Nguyen
https://yseali.fulbright.edu.vn/en/faculty/bicky-n...
The environmental footprint of beef production
https://www.earthsave.org/environment.htm
https://www.watercalculator.org/news/articles/beef-king-big-water-footprints/
https://www.frontiersin.org/articles/10.3389/fsufs.2019.00005/full
https://ourworldindata.org/carbon-footprint-food-methane
Insect farming as a source of sustainable protein
https://www.insectgourmet.com/insect-farming-growing-bugs-for-protein/
https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/insect-farming
Cricket flour is taking the world by storm
https://www.cricketflours.com/
https://talk-commerce.com/blog/what-brands-use-cricket-flour-and-why/

Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.