Deep Brain Stimulation for Mental Illnesses Raises Ethical Concerns
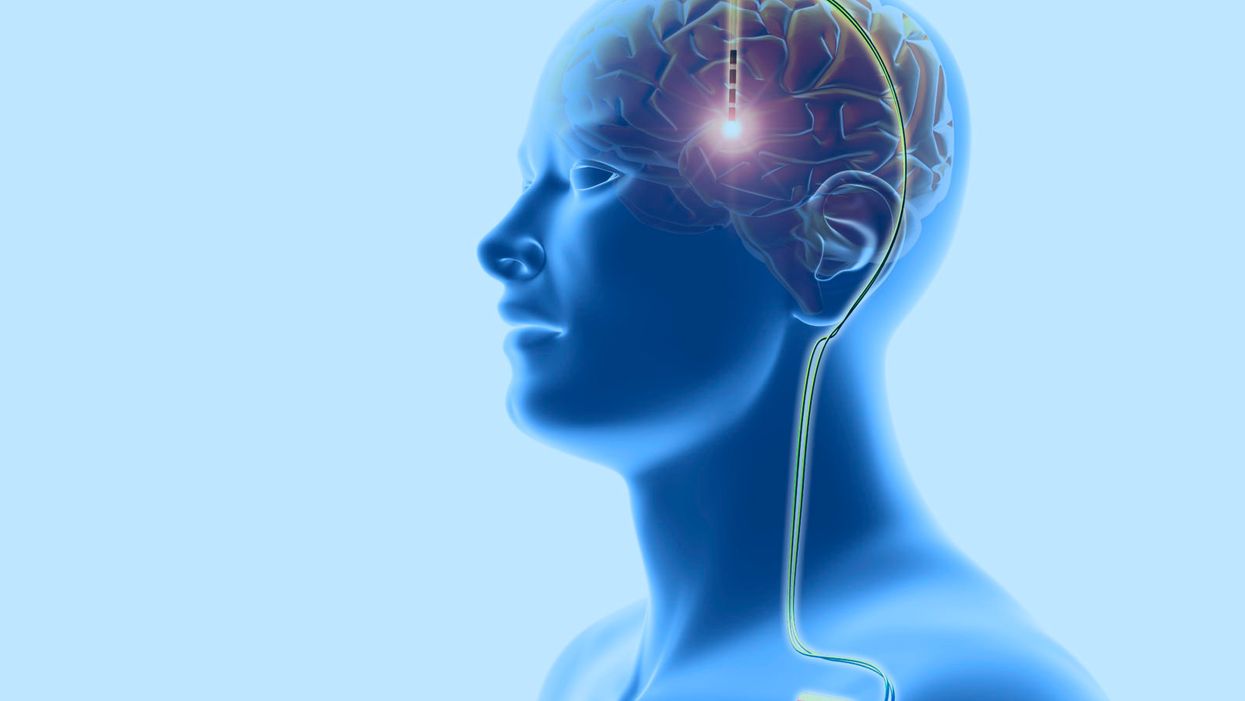
Deep brain stimulation: This neurosurgical treatment involves the implantation of electrodes in the cerebral lobes of the brain, linked through the scalp (top) to wires (down right) leading to a battery implanted below the skin. This sends electrical impulses to specific areas of the brain. DBS was developed for the treatment of Parkinson's disease, but is being investigated for use in other conditions.
Imagine that you are one of the hundreds of millions of people who suffer from depression. Medication hasn't helped you, so you're looking for another treatment option. Something powerful enough to change your mood as soon as you need a lift.
"If a participant experiences a personality change, does this change who they are or dehumanize them by altering their nature?"
Enter deep brain stimulation: a type of therapy in which one or more electrodes are inserted into your brain and connected to a surgically implanted, battery-operated medical device in your chest. This device, which is approximately the size of a stopwatch, sends electric pulses to a targeted region of your brain. The idea is to control a variety of neurological symptoms that can't be adequately managed by drugs.
Over the last twenty years, deep brain stimulation, known as DBS, has become an efficient and safe alternative for the treatment of chronic neurological diseases such as epilepsy, Parkinson's disease and neuropathic pain. According to the International Neuromodulation Society, there have been more than 80,000 deep brain stimulation implants performed around the world.
The Food and Drug Administration approved DBS as a treatment for essential tremor and Parkinson's in 1997, dystonia in 2003 and obsessive compulsive disorder in 2009. Since doctors can use drugs and treatments "off-label" (not approved by the FDA) to treat patients with any disease, DBS is now also being investigated as a treatment for chronic pain, PTSD and major depression.
And these new applications are raising profound ethical questions about individuality, personality, and even what it means to be human.
"These patients are essentially having a computer that can modify and influence emotional processing, mood and motor outputs inserted into the brain," said Gabriel Lazaro-Munoz, an assistant professor at The Center for Medical Ethics and Health Policy at Baylor College of Medicine. "These responses define us as human beings and dictate our autonomy. If a participant experiences a personality change, does this change who they are or dehumanize them by altering their nature? These are some of the questions we have to consider."
"When we are not in control of ourselves, are we ourselves?"
The U.S. government has similar concerns about DBS. The National Institutes of Health recently awarded grants to study the neuroethical issues surrounding the use of DBS in neuropsychiatric and movement disorders and appropriate consent for brain research. The grants are part of the Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative. Walter Koroshetz, director of NIH's National Institute of Neurological Disorders and Stroke said, "Neuroscience is rapidly moving toward a new frontier of research on human brains that may have long-lasting and unforeseen effects. These new awards signal our commitment to research conducted in a responsible way as to anticipate all potential consequences, and to ensure that research subjects have a clear understanding of the potential benefits and risks of participating in studies."
Dr. Lazaro-Munoz's Center was awarded one of the grants to identify and evaluate the ethical, legal and social concerns with adaptive deep brain stimulation (aDBS) technologies. Adaptive DBS is a relatively new version of the technology that enables recording of brain cell activity that is then used to regulate the brain in real time. He and his team will closely observe researchers conducting aDBS studies and administering in-depth interviews to trial participants, their caregivers, and researchers, as well as individuals who declined to participate in such studies. The goal is to gain a better understanding of the ethical concerns at stake in order to guide responsible research.
Dr. Lazaro-Munoz said one of the concerns is dehumanization. "By using this technology are we compromising what makes us human? When we are not in control of ourselves, are we ourselves?" He notes that similar concerns were raised about pharmaceutical treatments for illnesses. "Both change behaviors and emotional processing. However, there is a difference. Culturally we are more used to using drugs, not implanting devices into brain and computer interfaces. Many people think of it as science fiction."
The changes in behavior due to DBS can be dramatic, perhaps none more so than with Parkinson's disease; patients may see their chronic tremors suddenly vanish.
Pills for OCD and depression take longer than DBS to see significant improvement, sometimes months. "A DBS device is either on or off. And patients and families see changes immediately," Dr. Lazaro-Munoz said. "Family members are often startled by these changes, as are the patients." He's observed that patients feel more in control with pills because they can alter and "play" with the dose or even skip a dose.
The changes in behavior due to DBS can be dramatic, perhaps none more so than with Parkinson's disease; patients may see their chronic tremors suddenly vanish, like in this must-see video.
But surgical procedures to treat motor symptoms are also increasingly being implicated as a cause of behavioral changes, both positive and negative, in patients with Parkinson's. The personality changes reported in patients who undergo DBS include hypermania, pathological gambling, hypersexuality, impulsivity and aggressiveness. One patient who suffered from OCD fell in love with the music of Johnny Cash when his brain was stimulated. On the positive side, patients report memory enhancement.
One patient who is pleased with DBS is Greg Barstead, who was diagnosed with Parkinson's in 2003, when he was the president of Colonial Penn Life Insurance Company. He also has dystonia, which affects his neck and shoulders. Barstead said that DBS has been helpful for a range of symptoms: "My shoulder is a lot less stiff and my neck hurts less. And my tremors are under control. It is not perfect, as it doesn't relieve all the Parkinson's symptoms, but it does enough of a good job that both my wife and I are very happy I had DBS."
"We are not exactly sure what part of the brain causes depression. Doctors have not identified where to implant the device."
He said he hasn't noticed any personality changes, but noted that the disease itself can cause such changes. In fact, studies have shown that it can cause many psychiatric problems including depression and hallucinations. And, approximately a third of Parkinson's patients develop dementia.
Arthur L. Caplan, founding head of the Division of Medical Ethics at NYU School of Medicine, notes that unlike psychosurgery, DBS can be turned on and off and the device can be removed. "There are less ethical concerns around treating patients with Parkinson's disease than other illnesses because surgeons know exactly where to implant the device and have many years of experience with it," he said, adding that he is concerned about using DBS for other illnesses, such as depression. "We are not exactly sure what part of the brain causes depression. Doctors have not identified where to implant the device. And I would certainly not advocate its use in patients with mild depression."
Dr. Lazaro-Munoz said of the personality changes possible with DBS, physicians need to consider how the patients were functioning without it. "Patients who are candidates for DBS typically used many medications as well as psychotherapy before opting for DBS," he explained. "To me, the question is what is the net result of using this technology? Does the patient have regrets? Are the changes in personality significant or not? Although most DBS patients report being happy they underwent the procedure, some say they don't feel like themselves after DBS. Others feel they are more like themselves, especially if there are dramatic improvements in movement problems or relief of OCD symptoms."
And then there is the question of money. The costs of DBS are covered by most insurance companies and Medicare only for FDA-approved targets like Parkinson's. Off-label uses are not covered, at least for now.
Caplan reminds people that DBS devices are manufactured by companies that are interested in making money and the average cost per treatment is around $50,000. "I am interested in seeing DBS move forward," he said. "But we must be careful and not allow industry to make it go too fast, or be used on too many people, before we know it is effective."
Thanks to safety cautions from the COVID-19 pandemic, a strain of influenza has been completely eliminated.
If you were one of the millions who masked up, washed your hands thoroughly and socially distanced, pat yourself on the back—you may have helped change the course of human history.
Scientists say that thanks to these safety precautions, which were introduced in early 2020 as a way to stop transmission of the novel COVID-19 virus, a strain of influenza has been completely eliminated. This marks the first time in human history that a virus has been wiped out through non-pharmaceutical interventions, such as vaccines.
The flu shot, explained

Influenza viruses type A and B are responsible for the majority of human illnesses and the flu season.
Centers for Disease Control
For more than a decade, flu shots have protected against two types of the influenza virus–type A and type B. While there are four different strains of influenza in existence (A, B, C, and D), only strains A, B, and C are capable of infecting humans, and only A and B cause pandemics. In other words, if you catch the flu during flu season, you’re most likely sick with flu type A or B.
Flu vaccines contain inactivated—or dead—influenza virus. These inactivated viruses can’t cause sickness in humans, but when administered as part of a vaccine, they teach a person’s immune system to recognize and kill those viruses when they’re encountered in the wild.
Each spring, a panel of experts gives a recommendation to the US Food and Drug Administration on which strains of each flu type to include in that year’s flu vaccine, depending on what surveillance data says is circulating and what they believe is likely to cause the most illness during the upcoming flu season. For the past decade, Americans have had access to vaccines that provide protection against two strains of influenza A and two lineages of influenza B, known as the Victoria lineage and the Yamagata lineage. But this year, the seasonal flu shot won’t include the Yamagata strain, because the Yamagata strain is no longer circulating among humans.
How Yamagata Disappeared

Flu surveillance data from the Global Initiative on Sharing All Influenza Data (GISAID) shows that the Yamagata lineage of flu type B has not been sequenced since April 2020.
Nature
Experts believe that the Yamagata lineage had already been in decline before the pandemic hit, likely because the strain was naturally less capable of infecting large numbers of people compared to the other strains. When the COVID-19 pandemic hit, the resulting safety precautions such as social distancing, isolating, hand-washing, and masking were enough to drive the virus into extinction completely.
Because the strain hasn’t been circulating since 2020, the FDA elected to remove the Yamagata strain from the seasonal flu vaccine. This will mark the first time since 2012 that the annual flu shot will be trivalent (three-component) rather than quadrivalent (four-component).
Should I still get the flu shot?
The flu shot will protect against fewer strains this year—but that doesn’t mean we should skip it. Influenza places a substantial health burden on the United States every year, responsible for hundreds of thousands of hospitalizations and tens of thousands of deaths. The flu shot has been shown to prevent millions of illnesses each year (more than six million during the 2022-2023 season). And while it’s still possible to catch the flu after getting the flu shot, studies show that people are far less likely to be hospitalized or die when they’re vaccinated.
Another unexpected benefit of dropping the Yamagata strain from the seasonal vaccine? This will possibly make production of the flu vaccine faster, and enable manufacturers to make more vaccines, helping countries who have a flu vaccine shortage and potentially saving millions more lives.
After his grandmother’s dementia diagnosis, one man invented a snack to keep her healthy and hydrated.
Founder Lewis Hornby and his grandmother Pat, sampling Jelly Drops—an edible gummy containing water and life-saving electrolytes.
On a visit to his grandmother’s nursing home in 2016, college student Lewis Hornby made a shocking discovery: Dehydration is a common (and dangerous) problem among seniors—especially those that are diagnosed with dementia.
Hornby’s grandmother, Pat, had always had difficulty keeping up her water intake as she got older, a common issue with seniors. As we age, our body composition changes, and we naturally hold less water than younger adults or children, so it’s easier to become dehydrated quickly if those fluids aren’t replenished. What’s more, our thirst signals diminish naturally as we age as well—meaning our body is not as good as it once was in letting us know that we need to rehydrate. This often creates a perfect storm that commonly leads to dehydration. In Pat’s case, her dehydration was so severe she nearly died.
When Lewis Hornby visited his grandmother at her nursing home afterward, he learned that dehydration especially affects people with dementia, as they often don’t feel thirst cues at all, or may not recognize how to use cups correctly. But while dementia patients often don’t remember to drink water, it seemed to Hornby that they had less problem remembering to eat, particularly candy.

Hornby wanted to create a solution for elderly people who struggled keeping their fluid intake up. He spent the next eighteen months researching and designing a solution and securing funding for his project. In 2019, Hornby won a sizable grant from the Alzheimer’s Society, a UK-based care and research charity for people with dementia and their caregivers. Together, through the charity’s Accelerator Program, they created a bite-sized, sugar-free, edible jelly drop that looked and tasted like candy. The candy, called Jelly Drops, contained 95% water and electrolytes—important minerals that are often lost during dehydration. The final product launched in 2020—and was an immediate success. The drops were able to provide extra hydration to the elderly, as well as help keep dementia patients safe, since dehydration commonly leads to confusion, hospitalization, and sometimes even death.
Not only did Jelly Drops quickly become a favorite snack among dementia patients in the UK, but they were able to provide an additional boost of hydration to hospital workers during the pandemic. In NHS coronavirus hospital wards, patients infected with the virus were regularly given Jelly Drops to keep their fluid levels normal—and staff members snacked on them as well, since long shifts and personal protective equipment (PPE) they were required to wear often left them feeling parched.
In April 2022, Jelly Drops launched in the United States. The company continues to donate 1% of its profits to help fund Alzheimer’s research.

