Diagnosed by App: Medical Testing in the Palm of Your Hand

The shift to in-home medical testing through smartphone apps is expected to save patients time in receiving certain kinds of diagnoses, but raises some privacy concerns.
Urinary tract infections aren't life-threatening, but they can be excruciatingly painful and debilitating.
"Overnight, I'd be gripped by this searing pain and I can barely walk," says Ling Koh, a Los Angeles-based bioengineer. But short of going to the ER or urgent care, she'd have to suffer for a few days until she could get in to see her family doctor for an antibiotic prescription.
Smartphones are now able to do on-the-spot diagnostic tests that were previously only able to be performed in a lab.
No longer. Koh, who works for Scanwell Health, was instrumental in the development of the company's smartphone app that is FDA-cleared for urinary tract infection screening. It allows someone to test urine at home using a paper test strip — the same one used by doctors in ERs and labs. The phone app reads a scan card from the test kit that can analyze what's on the strip and then connect the patient to a physician who can make a virtual diagnosis.
Test strips cost $15 for a three-pack and consultation with a doc is about the same as an average co-pay -- $25, and the app matches the quality of clinical laboratory tests, according to the company. Right now, you can get a referral to a telehealth visit with a doctor in California and get a prescription. A national rollout is in the works within the next couple of months.
"It's so easy to use them at home and eliminate the inefficiencies in the process," says Koh. "A telemedicine doctor can look at the test results and prescribe directly to the pharmacy instead of women waiting at home, miserable, and crying in the bathtub."
Scanwell is now involved in an ongoing National Institutes of Health- sponsored study of chronic kidney disease to test a version of the app to identify patients who have the disease, which affects more than 30 million Americans. "Because kidney disease has virtually no symptoms, by the time people realize they're sick, their illness is advanced and they're ready for dialysis," says Koh. "If we can catch it sooner, early intervention can help them avoid kidney failure."
Smartphones have changed society — and now they may change medical care, too. Thanks to the incredible processing capabilities of our smartphones, which come equipped with a camera, access to the internet and are thousands of times faster than the 1960s era NASA computers that ran the Apollo Moon Mission, these pocket-sized powerhouses have become an invaluable tool for managing our health and are even able to do on-the-spot diagnostic tests that were previously only able to be performed in a lab.
This shift to in-home testing is the wave of the future, promising to ease some of the medical care bottlenecks in which patients can have two- to three-week waits to see their family doctors and lift some of the burdens on overworked physicians.
"This is really the democratization of medicine because a lot of the things we used to rely on doctors, hospitals, or labs to do we'll be able to do ourselves," says Dr. Eric Topol, an eminent cardiologist and digital health pioneer at the Scripps Clinic and Research Institute in La Jolla.
But troubling questions remain. Aside from the obvious convenience, are these tests truly as accurate as ones in a doctor's office? And with all this medical information stored and collected by smartphones, will privacy be sacrificed? Will friends, family members, and employers suddenly have access to personal medical information we'd rather keep to ourselves?
The range of what these DIY health care apps can do is mind-boggling, and even more complex tests are on the way.
"I'm really worried about that because we've let our guard down," says Topol. "Data stored on servers is a target for cyber thieves — and data is being breached, hacked, brokered, and sold, and we're complacent."
Still, the apps have come a long way since 2011 when Topol whipped out an experimental smartphone electro-cardiogram that he had been testing on his patients when a fellow passenger on a flight from Washington D.C. was seized with severe chest pains. At 35,000 feet in the air, the app, which uses fingertip sensors to detect heart rate, showed the man was having a heart attack. After an emergency landing, the passenger was rushed to the closest hospital and survived. These days, even the Apple Watch has an FDA-approved app that can monitor your electro-cardiogram readings.
The range of what these DIY health care apps can do is mind-boggling, and even more complex tests are on the way. Phone apps can now monitor sleep quality to detect sleep apnea, blood pressure, weight and temperature. In the future, rapid diagnostic tests for infectious diseases, like flu, Dengue or Zika, and urinalysis will become common.
"There is virtually no limit to the kinds of testing that can be done using a smartphone," says Dr. John Halamka, Executive Director of the Health Technology Exploration Center at Beth Israel Lahey Health. "No one wants to drive to a clinician's office or lab if that same quality testing can be achieved at a lower cost without leaving home."
SkinVision's skin cancer screening tool, for instance, can tell if a suspicious mole is cancerous. Users take three photos, which are then run through the app's algorithm that compares their lesions with more than three million pictures, evaluating such elements as asymmetry, color, and shape, and spits out an assessment within thirty seconds. A team of in-house experts provide a review regardless of whether the mole is high or low risk, and the app encourages users to see their doctors. The Dutch-based company's app has been used by more than a million people globally in the EU, and in New Zealand and Australia, where skin cancer is rampant and early detection can save lives. The company has plans to enter the U.S. market, according to a spokesperson.
Apps like Instant Heart Rate analyze blood flow, which can indicate whether your heart is functioning normally, while uChek examines urine samples for up to 10 markers for conditions like diabetes and urinary tract infections. Some behavioral apps even have sensors that can spot suicide risks if users are less active, indicating they may be suffering from a bout of the blues.
Even more complex tests are in the research pipeline. Apps like ResAppDX could eventually replace x-rays, CT scans, and blood tests in diagnosing severe respiratory infections in kids, while an EU-funded project called i-Prognosis can track a variety of clues — voice changes, facial expressions, hand steadiness — that indicate the onset of Parkinson's disease.
These hand-held testing devices can be especially helpful in developing countries, and there are pilot programs to use smartphone technology to diagnose malaria and HIV infections in remote outposts in Africa.
"In a lot of these places, there's no infrastructure but everyone has a smartphone," says Scanwell's Koh. "We need to leverage the smartphone in a clinically relevant way."
However, patient privacy is an ongoing concern. A 2019 review in the Journal of the American Medical Association conducted by Australian and American researchers looked at three dozen behavioral health apps, mainly for depression and smoking cessation. They found that about 70 percent shared data with third parties, like Facebook and Google, but only one third of them disclosed this in a privacy policy.
"Patients just blindly accept the end user agreements without understanding the implications."
Users need to be vigilant, too. "Patients just blindly accept the end user agreements without understanding the implications," says Hamalka, who is also the Chief Information Officer and Dean for Technology at Harvard Medical School.
And quality control is an issue. Right now, the diagnostic tools currently available have been vetted by the FDA, and overseas companies like Skin Vision have been scrutinized by the U.K.'s National Health Service and the EU. But the danger is that a lot of apps are going to be popping up soon that haven't been properly tested, due to loopholes in the regulations.
"All we want," says Topol, "are rigorous studies to make sure what consumers are using is validated."
[Correction, August 19th, 2019: An earlier version of this story misstated the specifics of SkinVision's service. A team of in-house experts reviews users' submissions, not in-house dermatologists, and the service is not free.]
Jamie Rettinger with his now fiance Amie Purnel-Davis, who helped him through the clinical trial.
Jamie Rettinger was still in his thirties when he first noticed a tiny streak of brown running through the thumbnail of his right hand. It slowly grew wider and the skin underneath began to deteriorate before he went to a local dermatologist in 2013. The doctor thought it was a wart and tried scooping it out, treating the affected area for three years before finally removing the nail bed and sending it off to a pathology lab for analysis.
"I have some bad news for you; what we removed was a five-millimeter melanoma, a cancerous tumor that often spreads," Jamie recalls being told on his return visit. "I'd never heard of cancer coming through a thumbnail," he says. None of his doctors had ever mentioned it either. "I just thought I was being treated for a wart." But nothing was healing and it continued to bleed.
A few months later a surgeon amputated the top half of his thumb. Lymph node biopsy tested negative for spread of the cancer and when the bandages finally came off, Jamie thought his medical issues were resolved.
Melanoma is the deadliest form of skin cancer. About 85,000 people are diagnosed with it each year in the U.S. and more than 8,000 die of the cancer when it spreads to other parts of the body, according to the Centers for Disease Control and Prevention (CDC).
There are two peaks in diagnosis of melanoma; one is in younger women ages 30-40 and often is tied to past use of tanning beds; the second is older men 60+ and is related to outdoor activity from farming to sports. Light-skinned people have a twenty-times greater risk of melanoma than do people with dark skin.
"When I graduated from medical school, in 2005, melanoma was a death sentence" --Diwakar Davar.
Jamie had a follow up PET scan about six months after his surgery. A suspicious spot on his lung led to a biopsy that came back positive for melanoma. The cancer had spread. Treatment with a monoclonal antibody (nivolumab/Opdivo®) didn't prove effective and he was referred to the UPMC Hillman Cancer Center in Pittsburgh, a four-hour drive from his home in western Ohio.
An alternative monoclonal antibody treatment brought on such bad side effects, diarrhea as often as 15 times a day, that it took more than a week of hospitalization to stabilize his condition. The only options left were experimental approaches in clinical trials.
Early research
"When I graduated from medical school, in 2005, melanoma was a death sentence" with a cure rate in the single digits, says Diwakar Davar, 39, an oncologist at UPMC Hillman Cancer Center who specializes in skin cancer. That began to change in 2010 with introduction of the first immunotherapies, monoclonal antibodies, to treat cancer. The antibodies attach to PD-1, a receptor on the surface of T cells of the immune system and on cancer cells. Antibody treatment boosted the melanoma cure rate to about 30 percent. The search was on to understand why some people responded to these drugs and others did not.
At the same time, there was a growing understanding of the role that bacteria in the gut, the gut microbiome, plays in helping to train and maintain the function of the body's various immune cells. Perhaps the bacteria also plays a role in shaping the immune response to cancer therapy.
One clue came from genetically identical mice. Animals ordered from different suppliers sometimes responded differently to the experiments being performed. That difference was traced to different compositions of their gut microbiome; transferring the microbiome from one animal to another in a process known as fecal transplant (FMT) could change their responses to disease or treatment.
When researchers looked at humans, they found that the patients who responded well to immunotherapies had a gut microbiome that looked like healthy normal folks, but patients who didn't respond had missing or reduced strains of bacteria.
Davar and his team knew that FMT had a very successful cure rate in treating the gut dysbiosis of Clostridioides difficile, a persistant intestinal infection, and they wondered if a fecal transplant from a patient who had responded well to cancer immunotherapy treatment might improve the cure rate of patients who did not originally respond to immunotherapies for melanoma.
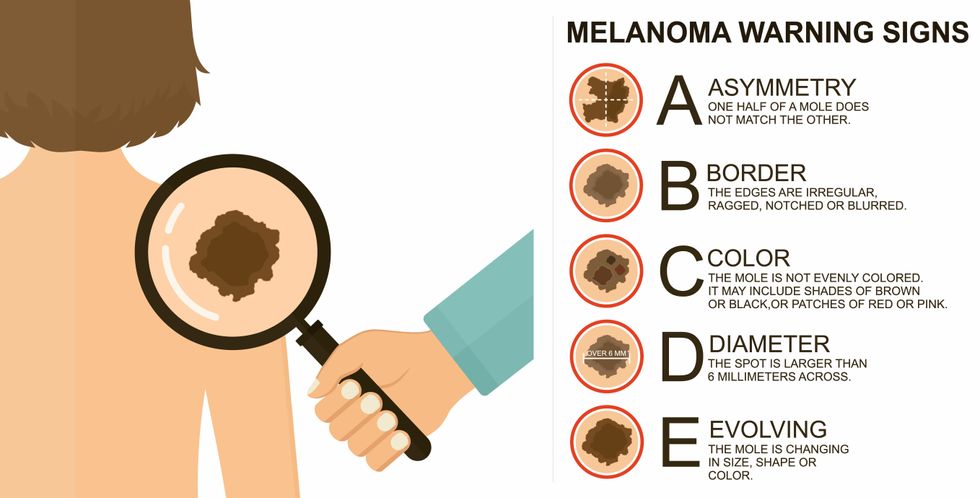
The ABCDE of melanoma detection
Adobe Stock
Clinical trial
"It was pretty weird, I was totally blasted away. Who had thought of this?" Jamie first thought when the hypothesis was explained to him. But Davar's explanation that the procedure might restore some of the beneficial bacterial his gut was lacking, convinced him to try. He quickly signed on in October 2018 to be the first person in the clinical trial.
Fecal donations go through the same safety procedures of screening for and inactivating diseases that are used in processing blood donations to make them safe for transfusion. The procedure itself uses a standard hollow colonoscope designed to screen for colon cancer and remove polyps. The transplant is inserted through the center of the flexible tube.
Most patients are sedated for procedures that use a colonoscope but Jamie doesn't respond to those drugs: "You can't knock me out. I was watching them on the TV going up my own butt. It was kind of unreal at that point," he says. "There were about twelve people in there watching because no one had seen this done before."
A test two weeks after the procedure showed that the FMT had engrafted and the once-missing bacteria were thriving in his gut. More importantly, his body was responding to another monoclonal antibody (pembrolizumab/Keytruda®) and signs of melanoma began to shrink. Every three months he made the four-hour drive from home to Pittsburgh for six rounds of treatment with the antibody drug.
"We were very, very lucky that the first patient had a great response," says Davar. "It allowed us to believe that even though we failed with the next six, we were on the right track. We just needed to tweak the [fecal] cocktail a little better" and enroll patients in the study who had less aggressive tumor growth and were likely to live long enough to complete the extensive rounds of therapy. Six of 15 patients responded positively in the pilot clinical trial that was published in the journal Science.
Davar believes they are beginning to understand the biological mechanisms of why some patients initially do not respond to immunotherapy but later can with a FMT. It is tied to the background level of inflammation produced by the interaction between the microbiome and the immune system. That paper is not yet published.
Surviving cancer
It has been almost a year since the last in his series of cancer treatments and Jamie has no measurable disease. He is cautiously optimistic that his cancer is not simply in remission but is gone for good. "I'm still scared every time I get my scans, because you don't know whether it is going to come back or not. And to realize that it is something that is totally out of my control."
"It was hard for me to regain trust" after being misdiagnosed and mistreated by several doctors he says. But his experience at Hillman helped to restore that trust "because they were interested in me, not just fixing the problem."
He is grateful for the support provided by family and friends over the last eight years. After a pause and a sigh, the ruggedly built 47-year-old says, "If everyone else was dead in my family, I probably wouldn't have been able to do it."
"I never hesitated to ask a question and I never hesitated to get a second opinion." But Jamie acknowledges the experience has made him more aware of the need for regular preventive medical care and a primary care physician. That person might have caught his melanoma at an earlier stage when it was easier to treat.
Davar continues to work on clinical studies to optimize this treatment approach. Perhaps down the road, screening the microbiome will be standard for melanoma and other cancers prior to using immunotherapies, and the FMT will be as simple as swallowing a handful of freeze-dried capsules off the shelf rather than through a colonoscopy. Earlier this year, the Food and Drug Administration approved the first oral fecal microbiota product for C. difficile, hopefully paving the way for more.
An older version of this hit article was first published on May 18, 2021
All organisms can repair damaged tissue, but none do it better than salamanders and newts. A surprising area of science could tell us how they manage this feat - and perhaps even help us develop a similar ability.
All organisms have the capacity to repair or regenerate tissue damage. None can do it better than salamanders or newts, which can regenerate an entire severed limb.
That feat has amazed and delighted man from the dawn of time and led to endless attempts to understand how it happens – and whether we can control it for our own purposes. An exciting new clue toward that understanding has come from a surprising source: research on the decline of cells, called cellular senescence.
Senescence is the last stage in the life of a cell. Whereas some cells simply break up or wither and die off, others transition into a zombie-like state where they can no longer divide. In this liminal phase, the cell still pumps out many different molecules that can affect its neighbors and cause low grade inflammation. Senescence is associated with many of the declining biological functions that characterize aging, such as inflammation and genomic instability.
Oddly enough, newts are one of the few species that do not accumulate senescent cells as they age, according to research over several years by Maximina Yun. A research group leader at the Center for Regenerative Therapies Dresden and the Max Planck Institute of Molecular and Cell Biology and Genetics, in Dresden, Germany, Yun discovered that senescent cells were induced at some stages of regeneration of the salamander limb, “and then, as the regeneration progresses, they disappeared, they were eliminated by the immune system,” she says. “They were present at particular times and then they disappeared.”
Senescent cells added to the edges of the wound helped the healthy muscle cells to “dedifferentiate,” essentially turning back the developmental clock of those cells into more primitive states.
Previous research on senescence in aging had suggested, logically enough, that applying those cells to the stump of a newly severed salamander limb would slow or even stop its regeneration. But Yun stood that idea on its head. She theorized that senescent cells might also play a role in newt limb regeneration, and she tested it by both adding and removing senescent cells from her animals. It turned out she was right, as the newt limbs grew back faster than normal when more senescent cells were included.
Senescent cells added to the edges of the wound helped the healthy muscle cells to “dedifferentiate,” essentially turning back the developmental clock of those cells into more primitive states, which could then be turned into progenitors, a cell type in between stem cells and specialized cells, needed to regrow the muscle tissue of the missing limb. “We think that this ability to dedifferentiate is intrinsically a big part of why salamanders can regenerate all these very complex structures, which other organisms cannot,” she explains.
Yun sees regeneration as a two part problem. First, the cells must be able to sense that their neighbors from the lost limb are not there anymore. Second, they need to be able to produce the intermediary progenitors for regeneration, , to form what is missing. “Molecularly, that must be encoded like a 3D map,” she says, otherwise the new tissue might grow back as a blob, or liver, or fin instead of a limb.
Wound healing
Another recent study, this time at the Mayo Clinic, provides evidence supporting the role of senescent cells in regeneration. Looking closely at molecules that send information between cells in the wound of a mouse, the researchers found that senescent cells appeared near the start of the healing process and then disappeared as healing progressed. In contrast, persistent senescent cells were the hallmark of a chronic wound that did not heal properly. The function and significance of senescence cells depended on both the timing and the context of their environment.
The paper suggests that senescent cells are not all the same. That has become clearer as researchers have been able to identify protein markers on the surface of some senescent cells. The patterns of these proteins differ for some senescent cells compared to others. In biology, such physical differences suggest functional differences, so it is becoming increasingly likely there are subsets of senescent cells with differing functions that have not yet been identified.
There are disagreements within the research community as to whether newts have acquired their regenerative capacity through a unique evolutionary change, or if other animals, including humans, retain this capacity buried somewhere in their genes.
Scientists initially thought that senescent cells couldn’t play a role in regeneration because they could no longer reproduce, says Anthony Atala, a practicing surgeon and bioengineer who leads the Wake Forest Institute for Regenerative Medicine in North Carolina. But Yun’s study points in the other direction. “What this paper shows clearly is that these cells have the potential to be involved in tissue regeneration [in newts]. The question becomes, will these cells be able to do the same in humans.”
As our knowledge of senescent cells increases, Atala thinks we need to embrace a new analogy to help understand them: humans in retirement. They “have acquired a lot of wisdom throughout their whole life and they can help younger people and mentor them to grow to their full potential. We're seeing the same thing with these cells,” he says. They are no longer putting energy into their own reproduction, but the signaling molecules they secrete “can help other cells around them to regenerate.”
There are disagreements within the research community as to whether newts have acquired their regenerative capacity through a unique evolutionary change, or if other animals, including humans, retain this capacity buried somewhere in their genes. If so, it seems that our genes are unable to express this ability, perhaps as part of a tradeoff in acquiring other traits. It is a fertile area of research.
Dedifferentiation is likely to become an important process in the field of regenerative medicine. One extreme example: a lab has been able to turn back the clock and reprogram adult male skin cells into female eggs, a potential milestone in reproductive health. It will be more difficult to control just how far back one wishes to go in the cell's dedifferentiation – part way or all the way back into a stem cell – and then direct it down a different developmental pathway. Yun is optimistic we can learn these tricks from newts.
Senolytics
A growing field of research is using drugs called senolytics to remove senescent cells and slow or even reverse disease of aging.
“Senolytics are great, but senolytics target different types of senescence,” Yun says. “If senescent cells have positive effects in the context of regeneration, of wound healing, then maybe at the beginning of the regeneration process, you may not want to take them out for a little while.”
“If you look at pretty much all biological systems, too little or too much of something can be bad, you have to be in that central zone” and at the proper time, says Atala. “That's true for proteins, sugars, and the drugs that you take. I think the same thing is true for these cells. Why would they be different?”
Our growing understanding that senescence is not a single thing but a variety of things likely means that effective senolytic drugs will not resemble a single sledge hammer but more a carefully manipulated scalpel where some types of senescent cells are removed while others are added. Combinations and timing could be crucial, meaning the difference between regenerating healthy tissue, a scar, or worse.

