How dozens of men across Alaska (and their dogs) teamed up to save one town from a deadly outbreak

In 1925, health officials in Alaska came up with a creative solution to save a remote fishing town from a deadly disease outbreak.
During the winter of 1924, Curtis Welch – the only doctor in Nome, a remote fishing town in northwest Alaska – started noticing something strange. More and more, the children of Nome were coming to his office with sore throats.
Initially, Welch dismissed the cases as tonsillitis or some run-of-the-mill virus – but when more kids started getting sick, with some even dying, he grew alarmed. It wasn’t until early 1925, after a three-year-old boy died just two weeks after becoming ill, that Welch realized that his worst suspicions were true. The boy – and dozens of other children in town – were infected with diphtheria.
A DEADLY BACTERIA
Diphtheria is nearly nonexistent and almost unheard of in industrialized countries today. But less than a century ago, diphtheria was a household name – one that struck fear in the heart of every parent, as it was extremely contagious and particularly deadly for children.
Diphtheria – a bacterial infection – is an ugly disease. When it strikes, the bacteria eats away at the healthy tissues in a patient’s respiratory tract, leaving behind a thick, gray membrane of dead tissue that covers the patient's nose, throat, and tonsils. Not only does this membrane make it very difficult for the patient to breathe and swallow, but as the bacteria spreads through the bloodstream, it causes serious harm to the heart and kidneys. It sometimes also results in nerve damage and paralysis. Even with treatment, diphtheria kills around 10 percent of people it infects. Young children, as well as adults over the age of 60, are especially at risk.
Welch didn’t suspect diphtheria at first. He knew the illness was incredibly contagious and reasoned that many more people would be sick – specifically, the family members of the children who had died – if there truly was an outbreak. Nevertheless, the symptoms, along with the growing number of deaths, were unmistakable. By 1925 Welch knew for certain that diphtheria had come to Nome.
In desperation, Welch tried treating an infected seven-year-old girl with some expired antitoxin – but she died just a few hours after he administered it.
AN INACCESSIBLE CURE
A vaccine for diphtheria wouldn’t be widely available until the mid-1930s and early 1940s – so an outbreak of the disease meant that each of the 10,000 inhabitants of Nome were all at serious risk.
One option was to use something called an antitoxin – a serum consisting of anti-diphtheria antibodies – to treat the patients. However, the town’s reserve of diphtheria antitoxin had expired. Welch had ordered a replacement shipment of antitoxin the previous summer – but the shipping port that was set to deliver the serum had been closed due to ice, and no new antitoxin would arrive before spring of 1925. In desperation, Welch tried treating an infected seven-year-old girl with some expired antitoxin – but she died just a few hours after he administered it.
Welch radioed for help to all the major towns in Alaska as well as the US Public Health Service in Washington, DC. His telegram read: An outbreak of diphtheria is almost inevitable here. I am in urgent need of one million units of diphtheria antitoxin. Mail is the only form of transportation.
FOUR-LEGGED HEROES
When the Alaskan Board of Health learned about the outbreak, the men rushed to devise a plan to get antitoxin to Nome. Dropping the serum in by airplane was impossible, as the available planes were unsuitable for flying during Alaska’s severe winter weather, where temperatures were routinely as cold as -50 degrees Fahrenheit.
In late January 1925, roughly 30,000 units of antitoxin were located in an Anchorage hospital and immediately delivered by train to a nearby city, Nenana, en route to Nome. Nenana was the furthest city that was reachable by rail – but unfortunately it was still more than 600 miles outside of Nome, with no transportation to make the delivery. Meanwhile, Welch had confirmed 20 total cases of diphtheria, with dozens more at high risk. Diphtheria was known for wiping out entire communities, and the entire town of Nome was in danger of suffering the same fate.
It was Mark Summer, the Board of Health superintendent, who suggested something unorthodox: Using a relay team of sled-racing dogs to deliver the antitoxin serum from Nenana to Nome. The Board quickly voted to accept Summer’s idea and set up a plan: The thousands of units of antitoxin serum would be passed along from team to team at different towns along the mail route from Nenana to Nome. When it reached a town called Nulato, a famed dogsled racer named Leonhard Seppala and his experienced team of huskies would take the serum more than 90 miles over the ice of Norton Sound, the longest and most treacherous part of the journey. Past the sound, the serum would change hands several times more before arriving in Nome.
Between January 27 and 31, the serum passed through roughly a dozen drivers and their dog sled teams, each of them carrying the serum between 20 and 50 miles to the next destination. Though each leg of the trip took less than a day, the sub-zero temperatures – sometimes as low as -85 degrees – meant that every driver and dog risked their lives. When the first driver, Bill Shannon, arrived at his checkpoint in Tolovana on January 28th, his nose was black with frostbite, and three of his dogs had died. The driver who relieved Bill Shannon, named Edgar Kalland, needed the owner of a local roadhouse to pour hot water over his hands to free them from the sled’s metal handlebar. Two more dogs from another relay team died before the serum was passed to Seppala at a town called Ungalik.
THE FINAL STRETCHES
Seppala and his team raced across the ice of the Norton Sound in the dead of night on January 31, with wind chill temperatures nearing an astonishing -90 degrees. The team traveled 84 miles in a single day before stopping to rest – and once rested, they set off again in the middle of the night through a raging winter storm. The team made it across the ice, as well as a 5,000-foot ascent up Little McKinley Mountain, to pass the serum to another driver in record time. The serum was now just 78 miles from Nome, and the death toll in town had reached 28.
The serum reached Gunnar Kaasen and his team of dogs on February 1st. Balto, Kaasen’s lead dog, guided the team heroically through a winter storm that was so severe Kaasen later reported not being able to see the dogs that were just a few feet ahead of him.
Visibility was so poor, in fact, that Kaasen ran his sled two miles past the relay point before noticing – and not wanting to lose a minute, he decided to forge on ahead rather than doubling back to deliver the serum to another driver. As they continued through the storm, the hurricane-force winds ripped past Kaasen’s sled at one point and toppled the sled – and the serum – overboard. The cylinder containing the antitoxin was left buried in the snow – and Kaasen tore off his gloves and dug through the tundra to locate it. Though it resulted in a bad case of frostbite, Kaasen eventually found the cylinder and kept driving.
Kaasen arrived at the next relay point on February 2nd, hours ahead of schedule. When he got there, however, he found the relay driver of the next team asleep. Kaasen took a risk and decided not to wake him, fearing that time would be wasted with the next driver readying his team. Kaasen, Balto, and the rest of the team forged on, driving another 25 miles before finally reaching Nome just before six in the morning. Eyewitnesses described Kaasen pulling up to the town’s bank and stumbling to the front of the sled. There, he collapsed in exhaustion, telling onlookers that Balto was “a damn fine dog.”
A LIVING LEGACY
Just a few hours after Balto’s heroic arrival in Nome, the serum had been thawed and was ready to administer to the patients with diphtheria. Amazingly, the relay team managed to complete the entire journey in just 127 hours – a world record at the time – without one serum vial damaged or destroyed. The serum shipment that arrived by dogsled – along with additional serum deliveries that followed in the next several weeks – were successful in stopping the outbreak in its tracks.
Balto and several other dogs – including Togo, the lead dog on Seppala’s team – were celebrated as local heroes after the race. Balto died in 1933, while the last of the human serum runners died in 1999 – but their legacy lives on: In early 2021, an all-female team of healthcare workers made the news by braving the Alaskan winter to deliver COVID-19 vaccines to people in rural North Alaska, traveling by bobsled and snowmobile – a heroic journey, and one that would have been unthinkable had Balto, Togo, and the 1925 sled runners not first paved the way.
DNA gathered from animal poop helps protect wildlife
Alida de Flamingh and her team are collecting elephant dung. It holds a trove of information about animal health, diet and genetic diversity.
On the savannah near the Botswana-Zimbabwe border, elephants grazed contentedly. Nearby, postdoctoral researcher Alida de Flamingh watched and waited. As the herd moved away, she went into action, collecting samples of elephant dung that she and other wildlife conservationists would study in the months to come. She pulled on gloves, took a swab, and ran it all over the still-warm, round blob of elephant poop.
Sequencing DNA from fecal matter is a safe, non-invasive way to track and ultimately help protect over 42,000 species currently threatened by extinction. Scientists are using this DNA to gain insights into wildlife health, genetic diversity and even the broader environment. Applied to elephants, chimpanzees, toucans and other species, it helps scientists determine the genetic diversity of groups and linkages with other groups. Such analysis can show changes in rates of inbreeding. Populations with greater genetic diversity adapt better to changes and environmental stressors than those with less diversity, thus reducing their risks of extinction, explains de Flamingh, a postdoctoral researcher at the University of Illinois Urbana-Champaign.
Analyzing fecal DNA also reveals information about an animal’s diet and health, and even nearby flora that is eaten. That information gives scientists broader insights into the ecosystem, and the findings are informing conservation initiatives. Examples include restoring or maintaining genetic connections among groups, ensuring access to certain foraging areas or increasing diversity in captive breeding programs.
Approximately 27 percent of mammals and 28 percent of all assessed species are close to dying out. The IUCN Red List of threatened species, simply called the Red List, is the world’s most comprehensive record of animals’ risk of extinction status. The more information scientists gather, the better their chances of reducing those risks. In Africa, populations of vertebrates declined 69 percent between 1970 and 2022, according to the World Wildlife Fund (WWF).
“We put on sterile gloves and use a sterile swab to collect wet mucus and materials from the outside of the dung ball,” says Alida de Flamingh, a postdoctoral researcher at the University of Illinois Urbana-Champaign.
“When people talk about species, they often talk about ecosystems, but they often overlook genetic diversity,” says Christina Hvilsom, senior geneticist at the Copenhagen Zoo. “It’s easy to count (individuals) to assess whether the population size is increasing or decreasing, but diversity isn’t something we can see with our bare eyes. Yet, it’s actually the foundation for the species and populations.” DNA analysis can provide this critical information.
Assessing elephants’ health
“Africa’s elephant populations are facing unprecedented threats,” says de Flamingh, the postdoc, who has studied them since 2009. Challenges include ivory poaching, habitat destruction and smaller, more fragmented habitats that result in smaller mating pools with less genetic diversity. Additionally, de Flamingh studies the microbial communities living on and in elephants – their microbiomes – looking for parasites or dangerous microbes.
Approximately 415,000 elephants inhabit Africa today, but de Flamingh says the number would be four times higher without these challenges. The IUCN Red List reports African savannah elephants are endangered and African forest elephants are critically endangered. Elephants support ecosystem biodiversity by clearing paths that help other species travel. Their very footprints create small puddles that can host smaller organisms such as tadpoles. Elephants are often described as ecosystems’ engineers, so if they disappear, the rest of the ecosystem will suffer too.
There’s a process to collecting elephant feces. “We put on sterile gloves (which we change for each sample) and use a sterile swab to collect wet mucus and materials from the outside of the dung ball,” says de Flamingh. They rub a sample about the size of a U.S. quarter onto a paper card embedded with DNA preservation technology. Each card is air dried and stored in a packet of desiccant to prevent mold growth. This way, samples can be stored at room temperature indefinitely without the DNA degrading.
Earlier methods required collecting dung in bags, which needed either refrigeration or the addition of preservatives, or the riskier alternative of tranquilizing the animals before approaching them to draw blood samples. The ability to collect and sequence the DNA made things much easier and safer.
“Our research provides a way to assess elephant health without having to physically interact with elephants,” de Flamingh emphasizes. “We also keep track of the GPS coordinates of each sample so that we can create a map of the sampling locations,” she adds. That helps researchers correlate elephants’ health with geographic areas and their conditions.
Although de Flamingh works with elephants in the wild, the contributions of zoos in the United States and collaborations in South Africa (notably the late Professor Rudi van Aarde and the Conservation Ecology Research Unit at the University of Pretoria) were key in studying this method to ensure it worked, she points out.
Protecting chimpanzees
Genetic work with chimpanzees began about a decade ago. Hvilsom and her group at the Copenhagen Zoo analyzed DNA from nearly 1,000 fecal samples collected between 2003 and 2018 by a team of international researchers. The goal was to assess the status of the West African subspecies, which is critically endangered after rapid population declines. Of the four subspecies of chimpanzees, the West African subspecies is considered the most at-risk.
In total, the WWF estimates the numbers of chimpanzees inhabiting Africa’s forests and savannah woodlands at between 173,000 and 300,000. Poaching, disease and human-caused changes to their lands are their major risks.
By analyzing genetics obtained from fecal samples, Hvilsom estimated the chimpanzees’ population, ascertained their family relationships and mapped their migration routes.
“One of the threats is mining near the Nimba Mountains in Guinea,” a stronghold for the West African subspecies, Hvilsom says. The Nimba Mountains are a UNESCO World Heritage Site, but they are rich in iron ore, which is used to make the steel that is vital to the Asian construction boom. As she and colleagues wrote in a recent paper, “Many extractive industries are currently developing projects in chimpanzee habitat.”
Analyzing DNA allows researchers to identify individual chimpanzees more accurately than simply observing them, she says. Normally, field researchers would install cameras and manually inspect each picture to determine how many chimpanzees were in an area. But, Hvilsom says, “That’s very tricky. Chimpanzees move a lot and are fast, so it’s difficult to get clear pictures. Often, they find and destroy the cameras. Also, they live in large areas, so you need a lot of cameras.”
By analyzing genetics obtained from fecal samples, Hvilsom estimated the chimpanzees’ population, ascertained their family relationships and mapped their migration routes based upon DNA comparisons with other chimpanzee groups. The mining companies and builders are using this information to locate future roads where they won’t disrupt migration – a more effective solution than trying to build artificial corridors for wildlife.
“The current route cuts off communities of chimpanzees,” Hvilsom elaborates. That effectively prevents young adult chimps from joining other groups when the time comes, eventually reducing the currently-high levels of genetic diversity.
“The mining company helped pay for the genetics work,” Hvilsom says, “as part of its obligation to assess and monitor biodiversity and the effect of the mining in the area.”
Of 50 toucan subspecies, 11 are threatened or near-threatened with extinction because of deforestation and poaching.
Identifying toucan families
Feces aren't the only substance researchers draw DNA samples from. Jeffrey Coleman, a Ph.D. candidate at the University of Texas at Austin relies on blood tests for studying the genetic diversity of toucans---birds species native to Central America and nearby regions. They live in the jungles, where they hop among branches, snip fruit from trees, toss it in the air and catch it with their large beaks. “Toucans are beautiful, charismatic birds that are really important to the ecosystem,” says Coleman.
Of their 50 subspecies, 11 are threatened or near-threatened with extinction because of deforestation and poaching. “When people see these aesthetically pleasing birds, they’re motivated to care about conservation practices,” he points out.
Coleman works with the Dallas World Aquarium and its partner zoos to analyze DNA from blood draws, using it to identify which toucans are related and how closely. His goal is to use science to improve the genetic diversity among toucan offspring.
Specifically, he’s looking at sections of the genome of captive birds in which the nucleotides repeat multiple times, such as AGATAGATAGAT. Called microsatellites, these consecutively-repeating sections can be passed from parents to children, helping scientists identify parent-child and sibling-sibling relationships. “That allows you to make strategic decisions about how to pair (captive) individuals for mating...to avoid inbreeding,” Coleman says.
Jeffrey Coleman is studying the microsatellites inside the toucan genomes.
Courtesy Jeffrey Coleman
The alternative is to use a type of analysis that looks for a single DNA building block – a nucleotide – that differs in a given sequence. Called single nucleotide polymorphisms (SNPs, pronounced “snips”), they are very common and very accurate. Coleman says they are better than microsatellites for some uses. But scientists have already developed a large body of microsatellite data from multiple species, so microsatellites can shed more insights on relations.
Regardless of whether conservation programs use SNPs or microsatellites to guide captive breeding efforts, the goal is to help them build genetically diverse populations that eventually may supplement endangered populations in the wild. “The hope is that the ecosystem will be stable enough and that the populations (once reintroduced into the wild) will be able to survive and thrive,” says Coleman. History knows some good examples of captive breeding success.
The California condor, which had a total population of 27 in 1987, when the last wild birds were captured, is one of them. A captive breeding program boosted their numbers to 561 by the end of 2022. Of those, 347 of those are in the wild, according to the National Park Service.
Conservationists hope that their work on animals’ genetic diversity will help preserve and restore endangered species in captivity and the wild. DNA analysis is crucial to both types of efforts. The ability to apply genome sequencing to wildlife conservation brings a new level of accuracy that helps protect species and gives fresh insights that observation alone can’t provide.
“A lot of species are threatened,” Coleman says. “I hope this research will be a resource people can use to get more information on longer-term genealogies and different populations.”
DNA- and RNA-based electronic implants may revolutionize healthcare
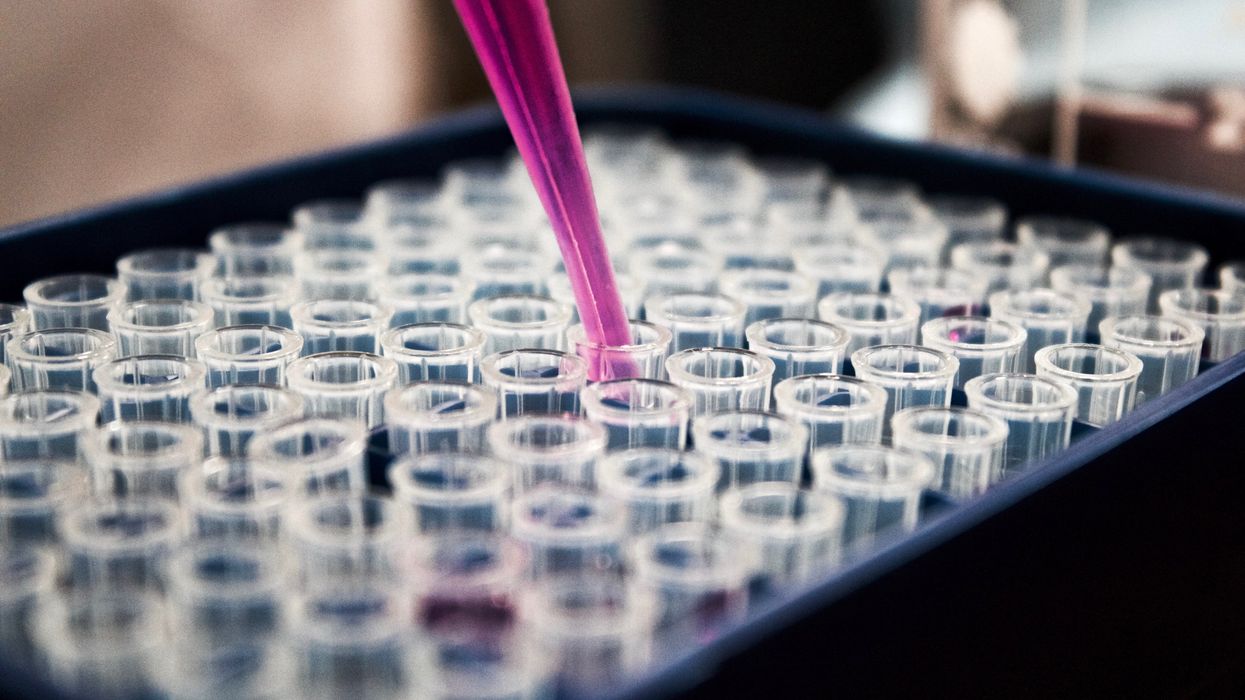
The test tubes contain tiny DNA/enzyme-based circuits, which comprise TRUMPET, a new type of electronic device, smaller than a cell.
Implantable electronic devices can significantly improve patients’ quality of life. A pacemaker can encourage the heart to beat more regularly. A neural implant, usually placed at the back of the skull, can help brain function and encourage higher neural activity. Current research on neural implants finds them helpful to patients with Parkinson’s disease, vision loss, hearing loss, and other nerve damage problems. Several of these implants, such as Elon Musk’s Neuralink, have already been approved by the FDA for human use.
Yet, pacemakers, neural implants, and other such electronic devices are not without problems. They require constant electricity, limited through batteries that need replacements. They also cause scarring. “The problem with doing this with electronics is that scar tissue forms,” explains Kate Adamala, an assistant professor of cell biology at the University of Minnesota Twin Cities. “Anytime you have something hard interacting with something soft [like muscle, skin, or tissue], the soft thing will scar. That's why there are no long-term neural implants right now.” To overcome these challenges, scientists are turning to biocomputing processes that use organic materials like DNA and RNA. Other promised benefits include “diagnostics and possibly therapeutic action, operating as nanorobots in living organisms,” writes Evgeny Katz, a professor of bioelectronics at Clarkson University, in his book DNA- And RNA-Based Computing Systems.
While a computer gives these inputs in binary code or "bits," such as a 0 or 1, biocomputing uses DNA strands as inputs, whether double or single-stranded, and often uses fluorescent RNA as an output.
Adamala’s research focuses on developing such biocomputing systems using DNA, RNA, proteins, and lipids. Using these molecules in the biocomputing systems allows the latter to be biocompatible with the human body, resulting in a natural healing process. In a recent Nature Communications study, Adamala and her team created a new biocomputing platform called TRUMPET (Transcriptional RNA Universal Multi-Purpose GatE PlaTform) which acts like a DNA-powered computer chip. “These biological systems can heal if you design them correctly,” adds Adamala. “So you can imagine a computer that will eventually heal itself.”
The basics of biocomputing
Biocomputing and regular computing have many similarities. Like regular computing, biocomputing works by running information through a series of gates, usually logic gates. A logic gate works as a fork in the road for an electronic circuit. The input will travel one way or another, giving two different outputs. An example logic gate is the AND gate, which has two inputs (A and B) and two different results. If both A and B are 1, the AND gate output will be 1. If only A is 1 and B is 0, the output will be 0 and vice versa. If both A and B are 0, the result will be 0. While a computer gives these inputs in binary code or "bits," such as a 0 or 1, biocomputing uses DNA strands as inputs, whether double or single-stranded, and often uses fluorescent RNA as an output. In this case, the DNA enters the logic gate as a single or double strand.
If the DNA is double-stranded, the system “digests” the DNA or destroys it, which results in non-fluorescence or “0” output. Conversely, if the DNA is single-stranded, it won’t be digested and instead will be copied by several enzymes in the biocomputing system, resulting in fluorescent RNA or a “1” output. And the output for this type of binary system can be expanded beyond fluorescence or not. For example, a “1” output might be the production of the enzyme insulin, while a “0” may be that no insulin is produced. “This kind of synergy between biology and computation is the essence of biocomputing,” says Stephanie Forrest, a professor and the director of the Biodesign Center for Biocomputing, Security and Society at Arizona State University.
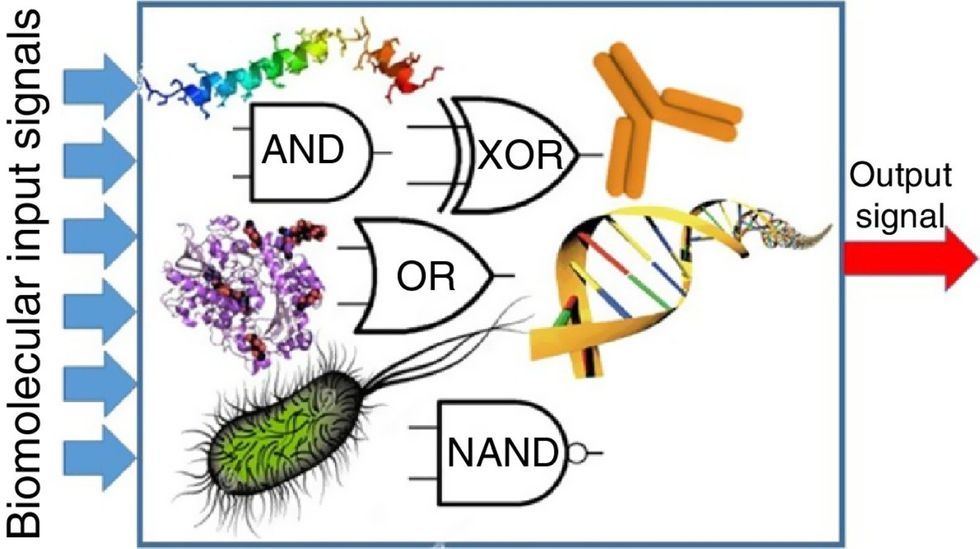
Biocomputing circles are made of DNA, RNA, proteins and even bacteria.
Evgeny Katz
The TRUMPET’s promise
Depending on whether the biocomputing system is placed directly inside a cell within the human body, or run in a test-tube, different environmental factors play a role. When an output is produced inside a cell, the cell's natural processes can amplify this output (for example, a specific protein or DNA strand), creating a solid signal. However, these cells can also be very leaky. “You want the cells to do the thing you ask them to do before they finish whatever their businesses, which is to grow, replicate, metabolize,” Adamala explains. “However, often the gate may be triggered without the right inputs, creating a false positive signal. So that's why natural logic gates are often leaky." While biocomputing outside a cell in a test tube can allow for tighter control over the logic gates, the outputs or signals cannot be amplified by a cell and are less potent.
TRUMPET, which is smaller than a cell, taps into both cellular and non-cellular biocomputing benefits. “At its core, it is a nonliving logic gate system,” Adamala states, “It's a DNA-based logic gate system. But because we use enzymes, and the readout is enzymatic [where an enzyme replicates the fluorescent RNA], we end up with signal amplification." This readout means that the output from the TRUMPET system, a fluorescent RNA strand, can be replicated by nearby enzymes in the platform, making the light signal stronger. "So it combines the best of both worlds,” Adamala adds.
These organic-based systems could detect cancer cells or low insulin levels inside a patient’s body.
The TRUMPET biocomputing process is relatively straightforward. “If the DNA [input] shows up as single-stranded, it will not be digested [by the logic gate], and you get this nice fluorescent output as the RNA is made from the single-stranded DNA, and that's a 1,” Adamala explains. "And if the DNA input is double-stranded, it gets digested by the enzymes in the logic gate, and there is no RNA created from the DNA, so there is no fluorescence, and the output is 0." On the story's leading image above, if the tube is "lit" with a purple color, that is a binary 1 signal for computing. If it's "off" it is a 0.
While still in research, TRUMPET and other biocomputing systems promise significant benefits to personalized healthcare and medicine. These organic-based systems could detect cancer cells or low insulin levels inside a patient’s body. The study’s lead author and graduate student Judee Sharon is already beginning to research TRUMPET's ability for earlier cancer diagnoses. Because the inputs for TRUMPET are single or double-stranded DNA, any mutated or cancerous DNA could theoretically be detected from the platform through the biocomputing process. Theoretically, devices like TRUMPET could be used to detect cancer and other diseases earlier.
Adamala sees TRUMPET not only as a detection system but also as a potential cancer drug delivery system. “Ideally, you would like the drug only to turn on when it senses the presence of a cancer cell. And that's how we use the logic gates, which work in response to inputs like cancerous DNA. Then the output can be the production of a small molecule or the release of a small molecule that can then go and kill what needs killing, in this case, a cancer cell. So we would like to develop applications that use this technology to control the logic gate response of a drug’s delivery to a cell.”
Although platforms like TRUMPET are making progress, a lot more work must be done before they can be used commercially. “The process of translating mechanisms and architecture from biology to computing and vice versa is still an art rather than a science,” says Forrest. “It requires deep computer science and biology knowledge,” she adds. “Some people have compared interdisciplinary science to fusion restaurants—not all combinations are successful, but when they are, the results are remarkable.”