FDA, researchers work to make clinical trials more diverse

The U.S. population is becoming more diverse, but clinical trials don't reflect that, experts say. Some are focusing on recruiting minorities to participate in research.
Nestled in a predominately Hispanic neighborhood, a new mural outside Guadalupe Centers Middle School in Kansas City, Missouri imparts a powerful message: “Clinical Research Needs Representation.” The colorful portraits painted above those words feature four cancer survivors of different racial and ethnic backgrounds. Two individuals identify as Hispanic, one as African American and another as Native American.
One of the patients depicted in the mural is Kim Jones, a 51-year-old African American breast cancer survivor since 2012. She advocated for an African American friend who participated in several clinical trials for ovarian cancer. Her friend was diagnosed in an advanced stage at age 26 but lived nine more years, thanks to the trials testing new therapeutics. “They are definitely giving people a longer, extended life and a better quality of life,” said Jones, who owns a nail salon. And that’s the message the mural aims to send to the community: Clinical trials need diverse participants.
While racial and ethnic minority groups represent almost half of the U.S. population, the lack of diversity in clinical trials poses serious challenges. Limited awareness and access impede equitable representation, which is necessary to prove the safety and effectiveness of medical interventions across different groups.
A Yale University study on clinical trial diversity published last year in BMJ Medicine found that while 81 percent of trials testing the new cancer drugs approved by the U.S. Food and Drug Administration between 2012 and 2017 included women, only 23 percent included older adults and 5 percent fairly included racial and ethnic minorities. “It’s both a public health and social justice issue,” said Jennifer E. Miller, an associate professor of medicine at Yale School of Medicine. “We need to know how medicines and vaccines work for all clinically distinct groups, not just healthy young White males.” A recent JAMA Oncology editorial stresses out the need for legislation that would require diversity action plans for certain types of trials.
Ensuring meaningful representation of racial and ethnic minorities in clinical trials for regulated medical products is fundamental to public health.--FDA Commissioner Robert M. Califf.
But change is on the horizon. Last April, the FDA issued a new draft guidance encouraging industry to find ways to revamp recruitment into clinical trials. The announcement, which expanded on previous efforts, called for including more participants from underrepresented racial and ethnic segments of the population.
“The U.S. population has become increasingly diverse, and ensuring meaningful representation of racial and ethnic minorities in clinical trials for regulated medical products is fundamental to public health,” FDA commissioner Robert M. Califf, a physician, said in a statement. “Going forward, achieving greater diversity will be a key focus throughout the FDA to facilitate the development of better treatments and better ways to fight diseases that often disproportionately impact diverse communities. This guidance also further demonstrates how we support the Administration’s Cancer Moonshot goal of addressing inequities in cancer care, helping to ensure that every community in America has access to cutting-edge cancer diagnostics, therapeutics and clinical trials.”
Lola Fashoyin-Aje, associate director for Science and Policy to Address Disparities in the Oncology Center of Excellence at the FDA, said that the agency “has long held the view that clinical trial participants should reflect the clinical and demographic characteristics of the patients who will ultimately receive the drug once approved.” However, “numerous studies over many decades” have measured the extent of underrepresentation. One FDA analysis found that the proportion of White patients enrolled in U.S. clinical trials (88 percent) is much higher than their numbers in country's population. Meanwhile, the enrollment of African American and Native Hawaiian/American Indian and Alaskan Native patients is below their national numbers.
The FDA’s guidance is accelerating researchers’ efforts to be more inclusive of diverse groups in clinical trials, said Joyce Sackey, a clinical professor of medicine and associate dean at Stanford School of Medicine. Underrepresentation is “a huge issue,” she noted. Sackey is focusing on this in her role as the inaugural chief equity, diversity and inclusion officer at Stanford Medicine, which encompasses the medical school and two hospitals.
Until the early 1990s, Sackey pointed out, clinical trials were based on research that mainly included men, as investigators were concerned that women could become pregnant, which would affect the results. This has led to some unfortunate consequences, such as indications and dosages for drugs that cause more side effects in women due to biological differences. “We’ve made some progress in including women, but we have a long way to go in including people of different ethnic and racial groups,” she said.

A new mural outside Guadalupe Centers Middle School in Kansas City, Missouri, advocates for increasing diversity in clinical trials. Kim Jones, 51-year-old African American breast cancer survivor, is second on the left.
Artwork by Vania Soto. Photo by Megan Peters.
Among racial and ethnic minorities, distrust of clinical trials is deeply rooted in a history of medical racism. A prime example is the Tuskegee Study, a syphilis research experiment that started in 1932 and spanned 40 years, involving hundreds of Black men with low incomes without their informed consent. They were lured with inducements of free meals, health care and burial stipends to participate in the study undertaken by the U.S. Public Health Service and the Tuskegee Institute in Alabama.
By 1947, scientists had figured out that they could provide penicillin to help patients with syphilis, but leaders of the Tuskegee research failed to offer penicillin to their participants throughout the rest of the study, which lasted until 1972.
Opeyemi Olabisi, an assistant professor of medicine at Duke University Medical Center, aims to increase the participation of African Americans in clinical research. As a nephrologist and researcher, he is the principal investigator of a clinical trial focusing on the high rate of kidney disease fueled by two genetic variants of the apolipoprotein L1 (APOL1) gene in people of recent African ancestry. Individuals of this background are four times more likely to develop kidney failure than European Americans, with these two variants accounting for much of the excess risk, Olabisi noted.
The trial is part of an initiative, CARE and JUSTICE for APOL1-Mediated Kidney Disease, through which Olabisi hopes to diversify study participants. “We seek ways to engage African Americans by meeting folks in the community, providing accessible information and addressing structural hindrances that prevent them from participating in clinical trials,” Olabisi said. The researchers go to churches and community organizations to enroll people who do not visit academic medical centers, which typically lead clinical trials. Since last fall, the initiative has screened more than 250 African Americans in North Carolina for the genetic variants, he said.
Other key efforts are underway. “Breaking down barriers, including addressing access, awareness, discrimination and racism, and workforce diversity, are pivotal to increasing clinical trial participation in racial and ethnic minority groups,” said Joshua J. Joseph, assistant professor of medicine at the Ohio State University Wexner Medical Center. Along with the university’s colleges of medicine and nursing, researchers at the medical center partnered with the African American Male Wellness Agency, Genentech and Pfizer to host webinars soliciting solutions from almost 450 community members, civic representatives, health care providers, government organizations and biotechnology professionals in 25 states and five countries.
Their findings, published in February in the journal PLOS One, suggested that including incentives or compensation as part of the research budget at the institutional level may help resolve some issues that hinder racial and ethnic minorities from participating in clinical trials. Compared to other groups, more Blacks and Hispanics have jobs in service, production and transportation, the authors note. It can be difficult to get paid leave in these sectors, so employees often can’t join clinical trials during regular business hours. If more leaders of trials offer money for participating, that could make a difference.
Obstacles include geographic access, language and other communications issues, limited awareness of research options, cost and lack of trust.
Christopher Corsico, senior vice president of development at GSK, formerly GlaxoSmithKline, said the pharmaceutical company conducted a 17-year retrospective study on U.S. clinical trial diversity. “We are using epidemiology and patients most impacted by a particular disease as the foundation for all our enrollment guidance, including study diversity plans,” Corsico said. “We are also sharing our results and ideas across the pharmaceutical industry.”
Judy Sewards, vice president and head of clinical trial experience at Pfizer’s headquarters in New York, said the company has committed to achieving racially and ethnically diverse participation at or above U.S. census or disease prevalence levels (as appropriate) in all trials. “Today, barriers to clinical trial participation persist,” Sewards said. She noted that these obstacles include geographic access, language and other communications issues, limited awareness of research options, cost and lack of trust. “Addressing these challenges takes a village. All stakeholders must come together and work collaboratively to increase diversity in clinical trials.”
It takes a village indeed. Hope Krebill, executive director of the Masonic Cancer Alliance, the outreach network of the University of Kansas Cancer Center in Kansas City, which commissioned the mural, understood that well. So her team actively worked with their metaphorical “village.” “We partnered with the community to understand their concerns, knowledge and attitudes toward clinical trials and research,” said Krebill. “With that information, we created a clinical trials video and a social media campaign, and finally, the mural to encourage people to consider clinical trials as an option for care.”
Besides its encouraging imagery, the mural will also be informational. It will include a QR code that viewers can scan to find relevant clinical trials in their location, said Vania Soto, a Mexican artist who completed the rendition in late February. “I’m so honored to paint people that are survivors and are living proof that clinical trials worked for them,” she said.
Jones, the cancer survivor depicted in the mural, hopes the image will prompt people to feel more open to partaking in clinical trials. “Hopefully, it will encourage people to inquire about what they can do — how they can participate,” she said.
Scientists turn pee into power in Uganda
With conventional fuel cells as their model, researchers learned to use similar chemical reactions to make a fuel from microbes in pee.
At the edge of a dirt road flanked by trees and green mountains outside the town of Kisoro, Uganda, sits the concrete building that houses Sesame Girls School, where girls aged 11 to 19 can live, learn and, at least for a while, safely use a toilet. In many developing regions, toileting at night is especially dangerous for children. Without electrical power for lighting, kids may fall into the deep pits of the latrines through broken or unsteady floorboards. Girls are sometimes assaulted by men who hide in the dark.
For the Sesame School girls, though, bright LED lights, connected to tiny gadgets, chased the fears away. They got to use new, clean toilets lit by the power of their own pee. Some girls even used the light provided by the latrines to study.
Urine, whether animal or human, is more than waste. It’s a cheap and abundant resource. Each day across the globe, 8.1 billion humans make 4 billion gallons of pee. Cows, pigs, deer, elephants and other animals add more. By spending money to get rid of it, we waste a renewable resource that can serve more than one purpose. Microorganisms that feed on nutrients in urine can be used in a microbial fuel cell that generates electricity – or "pee power," as the Sesame girls called it.
Plus, urine contains water, phosphorus, potassium and nitrogen, the key ingredients plants need to grow and survive. Human urine could replace about 25 percent of current nitrogen and phosphorous fertilizers worldwide and could save water for gardens and crops. The average U.S. resident flushes a toilet bowl containing only pee and paper about six to seven times a day, which adds up to about 3,500 gallons of water down per year. Plus cows in the U.S. produce 231 gallons of the stuff each year.
Pee power
A conventional fuel cell uses chemical reactions to produce energy, as electrons move from one electrode to another to power a lightbulb or phone. Ioannis Ieropoulos, a professor and chair of Environmental Engineering at the University of Southampton in England, realized the same type of reaction could be used to make a fuel from microbes in pee.
Bacterial species like Shewanella oneidensis and Pseudomonas aeruginosa can consume carbon and other nutrients in urine and pop out electrons as a result of their digestion. In a microbial fuel cell, one electrode is covered in microbes, immersed in urine and kept away from oxygen. Another electrode is in contact with oxygen. When the microbes feed on nutrients, they produce the electrons that flow through the circuit from one electrod to another to combine with oxygen on the other side. As long as the microbes have fresh pee to chomp on, electrons keep flowing. And after the microbes are done with the pee, it can be used as fertilizer.
These microbes are easily found in wastewater treatment plants, ponds, lakes, rivers or soil. Keeping them alive is the easy part, says Ieropoulos. Once the cells start producing stable power, his group sequences the microbes and keeps using them.
Like many promising technologies, scaling these devices for mass consumption won’t be easy, says Kevin Orner, a civil engineering professor at West Virginia University. But it’s moving in the right direction. Ieropoulos’s device has shrunk from the size of about three packs of cards to a large glue stick. It looks and works much like a AAA battery and produce about the same power. By itself, the device can barely power a light bulb, but when stacked together, they can do much more—just like photovoltaic cells in solar panels. His lab has produced 1760 fuel cells stacked together, and with manufacturing support, there’s no theoretical ceiling, he says.
Although pure urine produces the most power, Ieropoulos’s devices also work with the mixed liquids of the wastewater treatment plants, so they can be retrofit into urban wastewater utilities.
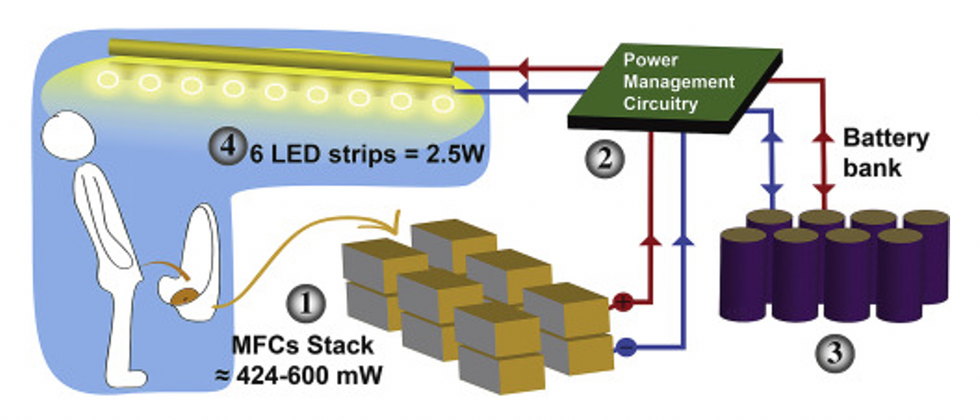
This image shows how the pee-powered system works. Pee feeds bacteria in the stack of fuel cells (1), which give off electrons (2) stored in parallel cylindrical cells (3). These cells are connected to a voltage regulator (4), which smooths out the electrical signal to ensure consistent power to the LED strips lighting the toilet.
Courtesy Ioannis Ieropoulos
Key to the long-term success of any urine reclamation effort, says Orner, is avoiding what he calls “parachute engineering”—when well-meaning scientists solve a problem with novel tech and then abandon it. “The way around that is to have either the need come from the community or to have an organization in a community that is committed to seeing a project operate and maintained,” he says.
Success with urine reclamation also depends on the economy. “If energy prices are low, it may not make sense to recover energy,” says Orner. “But right now, fertilizer prices worldwide are generally pretty high, so it may make sense to recover fertilizer and nutrients.” There are obstacles, too, such as few incentives for builders to incorporate urine recycling into new construction. And any hiccups like leaks or waste seepage will cost builders money and reputation. Right now, Orner says, the risks are just too high.
Despite the challenges, Ieropoulos envisions a future in which urine is passed through microbial fuel cells at wastewater treatment plants, retrofitted septic tanks, and building basements, and is then delivered to businesses to use as agricultural fertilizers. Although pure urine produces the most power, Ieropoulos’s devices also work with the mixed liquids of the wastewater treatment plants, so they can be retrofitted into urban wastewater utilities where they can make electricity from the effluent. And unlike solar cells, which are a common target of theft in some areas, nobody wants to steal a bunch of pee.
When Ieropoulos’s team returned to wrap up their pilot project 18 months later, the school’s director begged them to leave the fuel cells in place—because they made a major difference in students’ lives. “We replaced it with a substantial photovoltaic panel,” says Ieropoulos, They couldn’t leave the units forever, he explained, because of intellectual property reasons—their funders worried about theft of both the technology and the idea. But the photovoltaic replacement could be stolen, too, leaving the girls in the dark.
The story repeated itself at another school, in Nairobi, Kenya, as well as in an informal settlement in Durban, South Africa. Each time, Ieropoulos vowed to return. Though the pandemic has delayed his promise, he is resolute about continuing his work—it is a moral and legal obligation. “We've made a commitment to ourselves and to the pupils,” he says. “That's why we need to go back.”
Urine as fertilizer
Modern day industrial systems perpetuate the broken cycle of nutrients. When plants grow, they use up nutrients the soil. We eat the plans and excrete some of the nutrients we pass them into rivers and oceans. As a result, farmers must keep fertilizing the fields while our waste keeps fertilizing the waterways, where the algae, overfertilized with nitrogen, phosphorous and other nutrients grows out of control, sucking up oxygen that other marine species need to live. Few global communities remain untouched by the related challenges this broken chain create: insufficient clean water, food, and energy, and too much human and animal waste.
The Rich Earth Institute in Vermont runs a community-wide urine nutrient recovery program, which collects urine from homes and businesses, transports it for processing, and then supplies it as fertilizer to local farms.
One solution to this broken cycle is reclaiming urine and returning it back to the land. The Rich Earth Institute in Vermont is one of several organizations around the world working to divert and save urine for agricultural use. “The urine produced by an adult in one day contains enough fertilizer to grow all the wheat in one loaf of bread,” states their website.
Notably, while urine is not entirely sterile, it tends to harbor fewer pathogens than feces. That’s largely because urine has less organic matter and therefore less food for pathogens to feed on, but also because the urinary tract and the bladder have built-in antimicrobial defenses that kill many germs. In fact, the Rich Earth Institute says it’s safe to put your own urine onto crops grown for home consumption. Nonetheless, you’ll want to dilute it first because pee usually has too much nitrogen and can cause “fertilizer burn” if applied straight without dilution. Other projects to turn urine into fertilizer are in progress in Niger, South Africa, Kenya, Ethiopia, Sweden, Switzerland, The Netherlands, Australia, and France.
Eleven years ago, the Institute started a program that collects urine from homes and businesses, transports it for processing, and then supplies it as fertilizer to local farms. By 2021, the program included 180 donors producing over 12,000 gallons of urine each year. This urine is helping to fertilize hay fields at four partnering farms. Orner, the West Virginia professor, sees it as a success story. “They've shown how you can do this right--implementing it at a community level scale."
In this week's Friday Five, breathing this way may cut down on anxiety, a fasting regimen that could make you sick, this type of job makes men more virile, 3D printed hearts could save your life, and the role of metformin in preventing dementia.
The Friday Five covers five stories in research that you may have missed this week. There are plenty of controversies and troubling ethical issues in science – and we get into many of them in our online magazine – but this news roundup focuses on scientific creativity and progress to give you a therapeutic dose of inspiration headed into the weekend.
Here are the promising studies covered in this week's Friday Five, featuring interviews with Dr. David Spiegel, associate chair of psychiatry and behavioral sciences at Stanford, and Dr. Filip Swirski, professor of medicine and cardiology at the Icahn School of Medicine at Mount Sinai.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
Here are the promising studies covered in this week's Friday Five, featuring interviews with Dr. David Spiegel, associate chair of psychiatry and behavioral sciences at Stanford, and Dr. Filip Swirski, professor of medicine and cardiology at the Icahn School of Medicine at Mount Sinai.
- Breathing this way cuts down on anxiety*
- Could your fasting regimen make you sick?
- This type of job makes men more virile
- 3D printed hearts could save your life
- Yet another potential benefit of metformin
* This video with Dr. Andrew Huberman of Stanford shows exactly how to do the breathing practice.
This podcast originally aired on March 3, 2023.