An At-Home Contagiousness Test for COVID-19 Already Exists. Why Can’t We Use It?
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.

Bobby Brooke Herrera, the co-founder and CEO of e25Bio, demonstrates the company's rapid paper-strip test for detecting the coronavirus.
You're lying in bed late at night, the foggy swirl of the pandemic's 8th month just beginning to fall behind you, when you detect a slight tickle at the back of your throat.
"If half of people choose to use these tests every other day, then we can stop transmission faster than a vaccine can."
Suddenly fully awake, a jolt of panicked electricity races through your body. Has COVID-19 come for you? In the U.S., answering this simple question is incredibly difficult.
Now, you might have to wait for hours in line in your car to get a test for $100, only to find out your result 10-14 days later -- much too late to matter in stopping an outbreak. Due to such obstacles, a recent report in JAMA Internal Medicine estimated that 9 out of 10 infections in the U.S. are being missed.
But what if you could use a paper strip in the privacy of your own home, like a pregnancy test, and find out if you are contagious in real time?
e25 Bio, a small company in Cambridge, Mass., has already created such a test and it has been sitting on a lab bench, inaccessible, since April. It is an antigen test, which looks for proteins on the outside of a virus, and can deliver results in about 15 minutes. Also like an over-the-counter pregnancy test, e25 envisions its paper strips as a public health screening tool, rather than a definitive diagnostic test. People who see a positive result would be encouraged to then seek out a physician-administered, gold-standard diagnostic test: the more sensitive PCR.
Typically, hospitals and other health facilities rely on PCR tests to diagnose viruses. This test can detect small traces of genetic material that a virus leaves behind in the human body, which tells a clinician that the patient is either actively infected with or recently cleared that virus. PCR is quite sensitive, meaning that it is able to detect the presence of a virus' genetic material very accurately.
But although PCR is the gold-standard for diagnostics, it's also the most labor-intensive way to test for a virus and takes a relatively long time to produce results. That's not a good match for stopping super-spreader events during an unchecked pandemic. PCR is also not great at identifying the infected people when they are most at risk of potentially transmitting the virus to others.
That's because the viral threshold at which PCR can detect a positive result is so low, that it's actually too sensitive for the purposes of telling whether someone is contagious.
"The majority of time someone is PCR positive, those [genetic] remnants do not indicate transmissible virus," epidemiologist Michael Mina recently Tweeted. "They indicate remnants of a recently cleared infection."
To stop the chain of transmission for COVID-19, he says, "We need a more accurate test than PCR, that turns positive when someone is able to transmit."
In other words, we need a test that is better at detecting whether a person is contagious, as opposed to whether a small amount of virus can be detected in their nose or saliva. This kind of test is especially critical given the research showing that asymptomatic and pre-symptomatic people have high viral loads and are spreading the virus undetected.
The critical question for contagiousness testing, then, is how big a dose of SARS-CoV-2, the virus that causes COVID, does it take to infect most people? Researchers are still actively trying to answer this. As Angela Rasmussen, a coronavirus expert at Columbia University, told STAT: "We don't know the amount that is required to cause an infection, but it seems that it's probably not a really, really small amount, like measles."
Amesh Adalja, an infectious disease physician and a senior scholar at the Johns Hopkins University Center for Health Security, told LeapsMag: "It's still unclear what viral load is associated with contagiousness but it is biologically plausible that higher viral loads, in general, are associated with more efficient transmission especially in symptomatic individuals. In those without symptoms, however, the same relationship may not hold and this may be one of the reasons young children, despite their high viral loads, are not driving outbreaks."
"Antigen tests work best when there's high viral loads. They're catching people who are super spreaders."
Mina and colleagues estimate that widespread use of weekly cheap, rapid tests that are 100 times less sensitive than PCR tests would prevent outbreaks -- as long as the people who are positive self-isolate.
So why can't we buy e25Bio's test at a drugstore right now? Ironically, it's barred for the very reason that it's useful in the first place: Because it is not sensitive enough to satisfy the U.S. Food and Drug Administration, according to the company.
"We're ready to go," says Carlos-Henri Ferré, senior associate of operations and communications at e25. "We've applied to FDA, and now it's in their hands."
The problem, he said, is that the FDA is evaluating applications for antigen tests based on criteria for assessing diagnostics, like PCR, even when the tests serve a different purpose -- as a screening tool.
"Antigen tests work best when there's high viral loads," Ferré says. "They're catching people who are super spreaders, that are capable of continuing the spread of disease … FDA criteria is for diagnostics and not this."
FDA released guidance on July 29th -- 140 days into the pandemic -- recommending that at-home tests should perform with at least 80 percent sensitivity if ordered by prescription, and at least 90 percent sensitivity if purchased over the counter. "The danger of a false negative result is that it can contribute to the spread of COVID-19," according to an FDA spokesperson. "However, oversight of a health care professional who reviews the results, in combination with the patient's symptoms and uses their clinical judgment to recommend additional testing, if needed, among other things, can help mitigate some risks."
Crucially, the 90 percent sensitivity recommendation is judged upon comparison to PCR tests, meaning that if a PCR test is able to detect virus in 100 samples, the at-home antigen test would need to detect virus in at least 90 of those samples. Since antigen tests only detect high viral loads, frustrated critics like Mina say that such guidance is "unreasonable."
"The FDA at this moment is not understanding the true potential for wide-scale frequent testing. In some ways this is not their fault," Mina told LeapsMag. "The FDA does not have any remit to evaluate tests that fall outside of medical diagnostic testing. The proposal I have put forth is not about diagnostic testing (leave that for symptomatic cases reporting to their physician and getting PCR tests)....Daily rapid tests are not about diagnosing people and they are not about public health surveillance and they are not about passports to go to school, out to dinner or into the office. They are about reducing population-level transmission given a similar approach as vaccines."
A reasonable standard, he added, would be to follow the World Health Organization's Target Product Profiles, which are documents to help developers build desirable and minimally acceptable testing products. "A decent limit," Mina says, "is a 70% or 80% sensitivity (if they truly require sensitivity as a metric) to detect virus at Ct values less than 25. This coincides with detection of the most transmissible people, which is important."
(A Ct value is a type of measurement that corresponds inversely to the amount of viral load in a given sample. Researchers have found that Ct values of 13-17 indicate high viral load, whereas Ct values greater than 34 indicate a lack of infectious virus.)
"We believe this should be an at-home test, but [if FDA approval comes through] the first rollout is to do this in laboratories, hospitals, and clinics."
"We believe that population screening devices have an immediate place and use in helping beat the virus," says Ferré. "You can have a significant impact even with a test at 60% sensitivity if you are testing frequently."
When presented with criticism of its recommendations, the FDA indicated that it will not automatically deny any at-home test that fails to meet the 90 percent sensitivity guidance.
"FDA is always open to alternative proposals from developers, including strategies for serial testing with less sensitive tests," a spokesperson wrote in a statement. "For example, it is possible that overall sensitivity of the strategy could be considered cumulatively rather than based on one-time testing….In the case of a manufacturer with an at-home test that can only detect people with COVID-19 when they have a high viral load, we encourage them to talk with us so we can better understand their test, how they propose to use it, and the validation data they have collected to support that use."
However, the FDA's actions so far conflict with its stated openness. e25 ended up adding a step to the protocol in order to better meet FDA standards for sensitivity, but that extra step—sending samples to a laboratory for results—will undercut the test's ability to work as an at-home screening tool.
"We believe this should be an at-home test, but [if FDA approval comes through] the first rollout is to do this in laboratories, hospitals, and clinics," Ferré says.
According to the FDA, no test developers have approached them with a request for an emergency use authorization that proposes an alternate testing paradigm, such as serial testing, to mitigate test sensitivity below 80 percent.
From a scientific perspective, antigen tests like e25Bio's are not the only horse in the race for a simple rapid test with potential for at-home use. CRISPR technology has long been touted as fertile ground for diagnostics, and in an eerily prescient interview with LeapsMag in November, CRISPR pioneer Feng Zhang spoke of its potential application as an at-home diagnostic for an infectious disease specifically.
"I think in the long run it will be great to see this for, say, at-home disease testing, for influenza and other sorts of important public health [concerns]," he said in the fall. "To be able to get a readout at home, people can potentially quarantine themselves rather than traveling to a hospital and then carrying the risk of spreading that disease to other people as they get to the clinic."
Zhang's company Sherlock Biosciences is now working on scaled-up manufacturing of a test to detect SARS CoV-2. Mammoth Biosciences, which secured funding from the National Institutes of Health's Rapid Acceleration of Diagnostics program, is also working on a CRISPR diagnostic for SARS CoV-2. Both would check the box for rapid testing, but so far not for at-home testing, as they would also require laboratory infrastructure to provide results.
If any at-home tests can clear the regulatory hurdles, they would also need to be manufactured on a large scale and be cheap enough to entice people to actually use them. In the world of at-home diagnostics, pregnancy tests have become the sole mainstream victor because they're simple to use, small to carry, easy to interpret, and costs about seven or eight dollars at any ubiquitous store, like Target or Walmart. By comparison, the at-home COVID collection tests that don't even offer diagnostics—you send away your sample to an external lab—all cost over $100 to take just one time.
For the time being, the only available diagnostics for COVID require a lab or an expensive dedicated machine to process. This disconnect could prolong the world's worst health crisis in a century.
"Daily rapid tests have enormous potential to sever transmission chains and create herd effects similar to herd immunity," Mina says. "We all recognize that vaccines and infections can result in herd immunity when something around half of people are no longer susceptible.
"The same thing exists with these tests. These are the intervention to stop the virus. If half of people choose to use these tests every other day, then we can stop transmission faster than a vaccine can. The technology exists, the theory and mathematics back it up, the epidemiology is sound. There is no reason we are not approaching this as strongly as we would be approaching vaccines."
--Additional reporting by Julia Sklar
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
DNA- and RNA-based electronic implants may revolutionize healthcare
The test tubes contain tiny DNA/enzyme-based circuits, which comprise TRUMPET, a new type of electronic device, smaller than a cell.
Implantable electronic devices can significantly improve patients’ quality of life. A pacemaker can encourage the heart to beat more regularly. A neural implant, usually placed at the back of the skull, can help brain function and encourage higher neural activity. Current research on neural implants finds them helpful to patients with Parkinson’s disease, vision loss, hearing loss, and other nerve damage problems. Several of these implants, such as Elon Musk’s Neuralink, have already been approved by the FDA for human use.
Yet, pacemakers, neural implants, and other such electronic devices are not without problems. They require constant electricity, limited through batteries that need replacements. They also cause scarring. “The problem with doing this with electronics is that scar tissue forms,” explains Kate Adamala, an assistant professor of cell biology at the University of Minnesota Twin Cities. “Anytime you have something hard interacting with something soft [like muscle, skin, or tissue], the soft thing will scar. That's why there are no long-term neural implants right now.” To overcome these challenges, scientists are turning to biocomputing processes that use organic materials like DNA and RNA. Other promised benefits include “diagnostics and possibly therapeutic action, operating as nanorobots in living organisms,” writes Evgeny Katz, a professor of bioelectronics at Clarkson University, in his book DNA- And RNA-Based Computing Systems.
While a computer gives these inputs in binary code or "bits," such as a 0 or 1, biocomputing uses DNA strands as inputs, whether double or single-stranded, and often uses fluorescent RNA as an output.
Adamala’s research focuses on developing such biocomputing systems using DNA, RNA, proteins, and lipids. Using these molecules in the biocomputing systems allows the latter to be biocompatible with the human body, resulting in a natural healing process. In a recent Nature Communications study, Adamala and her team created a new biocomputing platform called TRUMPET (Transcriptional RNA Universal Multi-Purpose GatE PlaTform) which acts like a DNA-powered computer chip. “These biological systems can heal if you design them correctly,” adds Adamala. “So you can imagine a computer that will eventually heal itself.”
The basics of biocomputing
Biocomputing and regular computing have many similarities. Like regular computing, biocomputing works by running information through a series of gates, usually logic gates. A logic gate works as a fork in the road for an electronic circuit. The input will travel one way or another, giving two different outputs. An example logic gate is the AND gate, which has two inputs (A and B) and two different results. If both A and B are 1, the AND gate output will be 1. If only A is 1 and B is 0, the output will be 0 and vice versa. If both A and B are 0, the result will be 0. While a computer gives these inputs in binary code or "bits," such as a 0 or 1, biocomputing uses DNA strands as inputs, whether double or single-stranded, and often uses fluorescent RNA as an output. In this case, the DNA enters the logic gate as a single or double strand.
If the DNA is double-stranded, the system “digests” the DNA or destroys it, which results in non-fluorescence or “0” output. Conversely, if the DNA is single-stranded, it won’t be digested and instead will be copied by several enzymes in the biocomputing system, resulting in fluorescent RNA or a “1” output. And the output for this type of binary system can be expanded beyond fluorescence or not. For example, a “1” output might be the production of the enzyme insulin, while a “0” may be that no insulin is produced. “This kind of synergy between biology and computation is the essence of biocomputing,” says Stephanie Forrest, a professor and the director of the Biodesign Center for Biocomputing, Security and Society at Arizona State University.
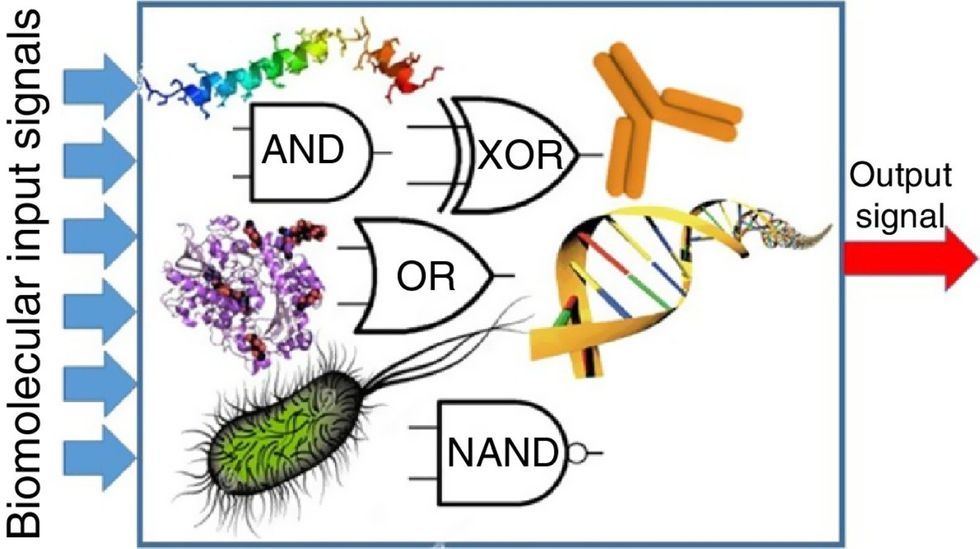
Biocomputing circles are made of DNA, RNA, proteins and even bacteria.
Evgeny Katz
The TRUMPET’s promise
Depending on whether the biocomputing system is placed directly inside a cell within the human body, or run in a test-tube, different environmental factors play a role. When an output is produced inside a cell, the cell's natural processes can amplify this output (for example, a specific protein or DNA strand), creating a solid signal. However, these cells can also be very leaky. “You want the cells to do the thing you ask them to do before they finish whatever their businesses, which is to grow, replicate, metabolize,” Adamala explains. “However, often the gate may be triggered without the right inputs, creating a false positive signal. So that's why natural logic gates are often leaky." While biocomputing outside a cell in a test tube can allow for tighter control over the logic gates, the outputs or signals cannot be amplified by a cell and are less potent.
TRUMPET, which is smaller than a cell, taps into both cellular and non-cellular biocomputing benefits. “At its core, it is a nonliving logic gate system,” Adamala states, “It's a DNA-based logic gate system. But because we use enzymes, and the readout is enzymatic [where an enzyme replicates the fluorescent RNA], we end up with signal amplification." This readout means that the output from the TRUMPET system, a fluorescent RNA strand, can be replicated by nearby enzymes in the platform, making the light signal stronger. "So it combines the best of both worlds,” Adamala adds.
These organic-based systems could detect cancer cells or low insulin levels inside a patient’s body.
The TRUMPET biocomputing process is relatively straightforward. “If the DNA [input] shows up as single-stranded, it will not be digested [by the logic gate], and you get this nice fluorescent output as the RNA is made from the single-stranded DNA, and that's a 1,” Adamala explains. "And if the DNA input is double-stranded, it gets digested by the enzymes in the logic gate, and there is no RNA created from the DNA, so there is no fluorescence, and the output is 0." On the story's leading image above, if the tube is "lit" with a purple color, that is a binary 1 signal for computing. If it's "off" it is a 0.
While still in research, TRUMPET and other biocomputing systems promise significant benefits to personalized healthcare and medicine. These organic-based systems could detect cancer cells or low insulin levels inside a patient’s body. The study’s lead author and graduate student Judee Sharon is already beginning to research TRUMPET's ability for earlier cancer diagnoses. Because the inputs for TRUMPET are single or double-stranded DNA, any mutated or cancerous DNA could theoretically be detected from the platform through the biocomputing process. Theoretically, devices like TRUMPET could be used to detect cancer and other diseases earlier.
Adamala sees TRUMPET not only as a detection system but also as a potential cancer drug delivery system. “Ideally, you would like the drug only to turn on when it senses the presence of a cancer cell. And that's how we use the logic gates, which work in response to inputs like cancerous DNA. Then the output can be the production of a small molecule or the release of a small molecule that can then go and kill what needs killing, in this case, a cancer cell. So we would like to develop applications that use this technology to control the logic gate response of a drug’s delivery to a cell.”
Although platforms like TRUMPET are making progress, a lot more work must be done before they can be used commercially. “The process of translating mechanisms and architecture from biology to computing and vice versa is still an art rather than a science,” says Forrest. “It requires deep computer science and biology knowledge,” she adds. “Some people have compared interdisciplinary science to fusion restaurants—not all combinations are successful, but when they are, the results are remarkable.”
Crickets are low on fat, high on protein, and can be farmed sustainably. They are also crunchy.
In today’s podcast episode, Leaps.org Deputy Editor Lina Zeldovich speaks about the health and ecological benefits of farming crickets for human consumption with Bicky Nguyen, who joins Lina from Vietnam. Bicky and her business partner Nam Dang operate an insect farm named CricketOne. Motivated by the idea of sustainable and healthy protein production, they started their unconventional endeavor a few years ago, despite numerous naysayers who didn’t believe that humans would ever consider munching on bugs.
Yet, making creepy crawlers part of our diet offers many health and planetary advantages. Food production needs to match the rise in global population, estimated to reach 10 billion by 2050. One challenge is that some of our current practices are inefficient, polluting and wasteful. According to nonprofit EarthSave.org, it takes 2,500 gallons of water, 12 pounds of grain, 35 pounds of topsoil and the energy equivalent of one gallon of gasoline to produce one pound of feedlot beef, although exact statistics vary between sources.
Meanwhile, insects are easy to grow, high on protein and low on fat. When roasted with salt, they make crunchy snacks. When chopped up, they transform into delicious pâtes, says Bicky, who invents her own cricket recipes and serves them at industry and public events. Maybe that’s why some research predicts that edible insects market may grow to almost $10 billion by 2030. Tune in for a delectable chat on this alternative and sustainable protein.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
Further reading:
More info on Bicky Nguyen
https://yseali.fulbright.edu.vn/en/faculty/bicky-n...
The environmental footprint of beef production
https://www.earthsave.org/environment.htm
https://www.watercalculator.org/news/articles/beef-king-big-water-footprints/
https://www.frontiersin.org/articles/10.3389/fsufs.2019.00005/full
https://ourworldindata.org/carbon-footprint-food-methane
Insect farming as a source of sustainable protein
https://www.insectgourmet.com/insect-farming-growing-bugs-for-protein/
https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/insect-farming
Cricket flour is taking the world by storm
https://www.cricketflours.com/
https://talk-commerce.com/blog/what-brands-use-cricket-flour-and-why/

Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.