Scientists find enzymes in nature that could replace toxic chemicals

Basecamp Research is using portable labs like this one to gather samples from ecosystems around the world.
Some 900 miles off the coast of Portugal, nine major islands rise from the mid-Atlantic. Verdant and volcanic, the Azores archipelago hosts a wealth of biodiversity that keeps field research scientist, Marlon Clark, returning for more. “You’ve got this really interesting biogeography out there,” says Clark. “There’s real separation between the continents, but there’s this inter-island dispersal of plants and seeds and animals.”
It’s a visual paradise by any standard, but on a microscopic level, there’s even more to see. The Azores’ nutrient-rich volcanic rock — and its network of lagoons, cave systems, and thermal springs — is home to a vast array of microorganisms found in a variety of microclimates with different elevations and temperatures.
Clark works for Basecamp Research, a biotech company headquartered in London, and his job is to collect samples from ecosystems around the world. By extracting DNA from soil, water, plants, microbes and other organisms, Basecamp is building an extensive database of the Earth’s proteins. While DNA itself isn’t a protein, the information stored in DNA is used to create proteins, so extracting, sequencing, and annotating DNA allows for the discovery of unique protein sequences.
Using what they’re finding in the middle of the Atlantic and beyond, Basecamp’s detailed database is constantly growing. The outputs could be essential for cleaning up the damage done by toxic chemicals and finding alternatives to these chemicals.
Catalysts for change
Proteins provide structure and function in all living organisms. Some of these functional proteins are enzymes, which quite literally make things happen.
“Industrial chemistry is heavily polluting, especially the chemistry done in pharmaceutical drug development. Biocatalysis is providing advantages, both to make more complex drugs and to be more sustainable, reducing the pollution and toxicity of conventional chemistry," says Ahir Pushpanath, who heads partnerships for Basecamp.
“Enzymes are perfectly evolved catalysts,” says Ahir Pushpanath, a partnerships lead at Basecamp. ”Enzymes are essentially just a polymer, and polymers are made up of amino acids, which are nature’s building blocks.” He suggests thinking about it like Legos — if you have a bunch of Lego pieces and use them to build a structure that performs a function, “that’s basically how an enzyme works. In nature, these monuments have evolved to do life’s chemistry. If we didn’t have enzymes, we wouldn’t be alive.”
In our own bodies, enzymes catalyze everything from vision to digesting food to regrowing muscles, and these same types of enzymes are necessary in the pharmaceutical, agrochemical and fine chemical industries. But industrial conditions differ from those inside our bodies. So, when scientists need certain chemical reactions to create a particular product or substance, they make their own catalysts in their labs — generally through the use of petroleum and heavy metals.
These petrochemicals are effective and cost-efficient, but they’re wasteful and often hazardous. With growing concerns around sustainability and long-term public health, it's essential to find alternative solutions to toxic chemicals. “Industrial chemistry is heavily polluting, especially the chemistry done in pharmaceutical drug development,” Pushpanath says.
Basecamp is trying to replace lab-created catalysts with enzymes found in the wild. This concept is called biocatalysis, and in theory, all scientists have to do is find the right enzymes for their specific need. Yet, historically, researchers have struggled to find enzymes to replace petrochemicals. When they can’t identify a suitable match, they turn to what Pushpanath describes as “long, iterative, resource-intensive, directed evolution” in the laboratory to coax a protein into industrial adaptation. But the latest scientific advances have enabled these discoveries in nature instead.

Marlon Clark, a research scientist at Basecamp Research, looks for novel biochemistries in the Azores.
Glen Gowers
Enzyme hunters
Whether it’s Clark and a colleague setting off on an expedition, or a local, on-the-ground partner gathering and processing samples, there’s a lot to be learned from each collection. “Microbial genomes contain complete sets of information that define an organism — much like how letters are a code allowing us to form words, sentences, pages, and books that contain complex but digestible knowledge,” Clark says. He thinks of the environmental samples as biological libraries, filled with thousands of species, strains, and sequence variants. “It’s our job to glean genetic information from these samples.”
“We can actually dream up new proteins using generative AI," Pushpanath says.
Basecamp researchers manage this feat by sequencing the DNA and then assembling the information into a comprehensible structure. “We’re building the ‘stories’ of the biota,” Clark says. The more varied the samples, the more valuable insights his team gains into the characteristics of different organisms and their interactions with the environment. Sequencing allows scientists to examine the order of nucleotides — the organic molecules that form DNA — to identify genetic makeups and find changes within genomes. The process used to be too expensive, but the cost of sequencing has dropped from $10,000 a decade ago to as low as $100. Notably, biocatalysis isn’t a new concept — there have been waves of interest in using natural enzymes in catalysis for over a century, Pushpanath says. “But the technology just wasn’t there to make it cost effective,” he explains. “Sequencing has been the biggest boon.”
AI is probably the second biggest boon.
“We can actually dream up new proteins using generative AI,” Pushpanath says, which means that biocataylsis now has real potential to scale.
Glen Gowers, the co-founder of Basecamp, compares the company’s AI approach to that of social networks and streaming services. Consider how these platforms suggest connecting with the friends of your friends, or how watching one comedy film from the 1990s leads to a suggestion of three more.
“They’re thinking about data as networks of relationships as opposed to lists of items,” says Gowers. “By doing the same, we’re able to link the metadata of the proteins — by their relationships to each other, the environments in which they’re found, the way those proteins might look similar in sequence and structure, their surrounding genome context — really, this just comes down to creating a searchable network of proteins.”

On an Azores island, this volcanic opening may harbor organisms that can help scientists identify enzymes for biocatalysis to replace toxic chemicals.
Emma Bolton
Uwe Bornscheuer, professor at the Institute of Biochemistry at the University of Greifswald, and co-founder of Enzymicals, another biocatalysis company, says that the development of machine learning is a critical component of this work. “It’s a very hot topic, because the challenge in protein engineering is to predict which mutation at which position in the protein will make an enzyme suitable for certain applications,” Bornscheuer explains. These predictions are difficult for humans to make at all, let alone quickly. “It is clear that machine learning is a key technology.”
Benefiting from nature’s bounty
Biodiversity commonly refers to plants and animals, but the term extends to all life, including microbial life, and some regions of the world are more biodiverse than others. Building relationships with global partners is another key element to Basecamp’s success. Doing so in accordance with the access and benefit sharing principles set forth by the Nagoya Protocol — an international agreement that seeks to ensure the benefits of using genetic resources are distributed in a fair and equitable way — is part of the company's ethos. “There's a lot of potential for us, and there’s a lot of potential for our partners to have exactly the same impact in building and discovering commercially relevant proteins and biochemistries from nature,” Clark says.
Bornscheuer points out that Basecamp is not the first company of its kind. A former San Diego company called Diversa went public in 2000 with similar work. “At that time, the Nagoya Protocol was not around, but Diversa also wanted to ensure that if a certain enzyme or microorganism from Costa Rica, for example, were used in an industrial process, then people in Costa Rica would somehow profit from this.”
An eventual merger turned Diversa into Verenium Corporation, which is now a part of the chemical producer BASF, but it laid important groundwork for modern companies like Basecamp to continue to scale with today’s technologies.
“To collect natural diversity is the key to identifying new catalysts for use in new applications,” Bornscheuer says. “Natural diversity is immense, and over the past 20 years we have gained the advantages that sequencing is no longer a cost or time factor.”
This has allowed Basecamp to rapidly grow its database, outperforming Universal Protein Resource or UniProt, which is the public repository of protein sequences most commonly used by researchers. Basecamp’s database is three times larger, totaling about 900 million sequences. (UniProt isn’t compliant with the Nagoya Protocol, because, as a public database, it doesn’t provide traceability of protein sequences. Some scientists, however, argue that Nagoya compliance hinders progress.)
“Eventually, this work will reduce chemical processes. We’ll have cleaner processes, more sustainable processes," says Uwe Bornscheuer, a professor at the University of Greifswald.
With so much information available, Basecamp’s AI has been trained on “the true dictionary of protein sequence life,” Pushpanath says, which makes it possible to design sequences for particular applications. “Through deep learning approaches, we’re able to find protein sequences directly from our database, without the need for further laboratory-directed evolution.”
Recently, a major chemical company was searching for a specific transaminase — an enzyme that catalyzes a transfer of amino groups. “They had already spent a year-and-a-half and nearly two million dollars to evolve a public-database enzyme, and still had not reached their goal,” Pushpanath says. “We used our AI approaches on our novel database to yield 10 candidates within a week, which, when validated by the client, achieved the desired target even better than their best-evolved candidate.”
Basecamp’s other huge potential is in bioremediation, where natural enzymes can help to undo the damage caused by toxic chemicals. “Biocatalysis impacts both sides,” says Gowers. “It reduces the usage of chemicals to make products, and at the same time, where contamination sites do exist from chemical spills, enzymes are also there to kind of mop those up.”
So far, Basecamp's round-the-world sampling has covered 50 percent of the 14 major biomes, or regions of the planet that can be distinguished by their flora, fauna, and climate, as defined by the World Wildlife Fund. The other half remains to be catalogued — a key milestone for understanding our planet’s protein diversity, Pushpanath notes.
There’s still a long road ahead to fully replace petrochemicals with natural enzymes, but biocatalysis is on an upward trajectory. "Eventually, this work will reduce chemical processes,” Bornscheuer says. “We’ll have cleaner processes, more sustainable processes.”
DNA- and RNA-based electronic implants may revolutionize healthcare
The test tubes contain tiny DNA/enzyme-based circuits, which comprise TRUMPET, a new type of electronic device, smaller than a cell.
Implantable electronic devices can significantly improve patients’ quality of life. A pacemaker can encourage the heart to beat more regularly. A neural implant, usually placed at the back of the skull, can help brain function and encourage higher neural activity. Current research on neural implants finds them helpful to patients with Parkinson’s disease, vision loss, hearing loss, and other nerve damage problems. Several of these implants, such as Elon Musk’s Neuralink, have already been approved by the FDA for human use.
Yet, pacemakers, neural implants, and other such electronic devices are not without problems. They require constant electricity, limited through batteries that need replacements. They also cause scarring. “The problem with doing this with electronics is that scar tissue forms,” explains Kate Adamala, an assistant professor of cell biology at the University of Minnesota Twin Cities. “Anytime you have something hard interacting with something soft [like muscle, skin, or tissue], the soft thing will scar. That's why there are no long-term neural implants right now.” To overcome these challenges, scientists are turning to biocomputing processes that use organic materials like DNA and RNA. Other promised benefits include “diagnostics and possibly therapeutic action, operating as nanorobots in living organisms,” writes Evgeny Katz, a professor of bioelectronics at Clarkson University, in his book DNA- And RNA-Based Computing Systems.
While a computer gives these inputs in binary code or "bits," such as a 0 or 1, biocomputing uses DNA strands as inputs, whether double or single-stranded, and often uses fluorescent RNA as an output.
Adamala’s research focuses on developing such biocomputing systems using DNA, RNA, proteins, and lipids. Using these molecules in the biocomputing systems allows the latter to be biocompatible with the human body, resulting in a natural healing process. In a recent Nature Communications study, Adamala and her team created a new biocomputing platform called TRUMPET (Transcriptional RNA Universal Multi-Purpose GatE PlaTform) which acts like a DNA-powered computer chip. “These biological systems can heal if you design them correctly,” adds Adamala. “So you can imagine a computer that will eventually heal itself.”
The basics of biocomputing
Biocomputing and regular computing have many similarities. Like regular computing, biocomputing works by running information through a series of gates, usually logic gates. A logic gate works as a fork in the road for an electronic circuit. The input will travel one way or another, giving two different outputs. An example logic gate is the AND gate, which has two inputs (A and B) and two different results. If both A and B are 1, the AND gate output will be 1. If only A is 1 and B is 0, the output will be 0 and vice versa. If both A and B are 0, the result will be 0. While a computer gives these inputs in binary code or "bits," such as a 0 or 1, biocomputing uses DNA strands as inputs, whether double or single-stranded, and often uses fluorescent RNA as an output. In this case, the DNA enters the logic gate as a single or double strand.
If the DNA is double-stranded, the system “digests” the DNA or destroys it, which results in non-fluorescence or “0” output. Conversely, if the DNA is single-stranded, it won’t be digested and instead will be copied by several enzymes in the biocomputing system, resulting in fluorescent RNA or a “1” output. And the output for this type of binary system can be expanded beyond fluorescence or not. For example, a “1” output might be the production of the enzyme insulin, while a “0” may be that no insulin is produced. “This kind of synergy between biology and computation is the essence of biocomputing,” says Stephanie Forrest, a professor and the director of the Biodesign Center for Biocomputing, Security and Society at Arizona State University.
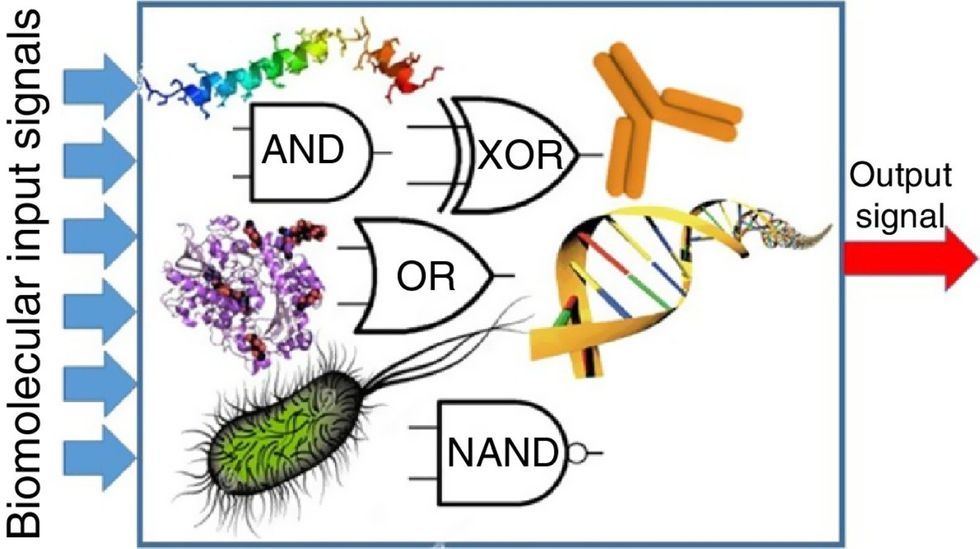
Biocomputing circles are made of DNA, RNA, proteins and even bacteria.
Evgeny Katz
The TRUMPET’s promise
Depending on whether the biocomputing system is placed directly inside a cell within the human body, or run in a test-tube, different environmental factors play a role. When an output is produced inside a cell, the cell's natural processes can amplify this output (for example, a specific protein or DNA strand), creating a solid signal. However, these cells can also be very leaky. “You want the cells to do the thing you ask them to do before they finish whatever their businesses, which is to grow, replicate, metabolize,” Adamala explains. “However, often the gate may be triggered without the right inputs, creating a false positive signal. So that's why natural logic gates are often leaky." While biocomputing outside a cell in a test tube can allow for tighter control over the logic gates, the outputs or signals cannot be amplified by a cell and are less potent.
TRUMPET, which is smaller than a cell, taps into both cellular and non-cellular biocomputing benefits. “At its core, it is a nonliving logic gate system,” Adamala states, “It's a DNA-based logic gate system. But because we use enzymes, and the readout is enzymatic [where an enzyme replicates the fluorescent RNA], we end up with signal amplification." This readout means that the output from the TRUMPET system, a fluorescent RNA strand, can be replicated by nearby enzymes in the platform, making the light signal stronger. "So it combines the best of both worlds,” Adamala adds.
These organic-based systems could detect cancer cells or low insulin levels inside a patient’s body.
The TRUMPET biocomputing process is relatively straightforward. “If the DNA [input] shows up as single-stranded, it will not be digested [by the logic gate], and you get this nice fluorescent output as the RNA is made from the single-stranded DNA, and that's a 1,” Adamala explains. "And if the DNA input is double-stranded, it gets digested by the enzymes in the logic gate, and there is no RNA created from the DNA, so there is no fluorescence, and the output is 0." On the story's leading image above, if the tube is "lit" with a purple color, that is a binary 1 signal for computing. If it's "off" it is a 0.
While still in research, TRUMPET and other biocomputing systems promise significant benefits to personalized healthcare and medicine. These organic-based systems could detect cancer cells or low insulin levels inside a patient’s body. The study’s lead author and graduate student Judee Sharon is already beginning to research TRUMPET's ability for earlier cancer diagnoses. Because the inputs for TRUMPET are single or double-stranded DNA, any mutated or cancerous DNA could theoretically be detected from the platform through the biocomputing process. Theoretically, devices like TRUMPET could be used to detect cancer and other diseases earlier.
Adamala sees TRUMPET not only as a detection system but also as a potential cancer drug delivery system. “Ideally, you would like the drug only to turn on when it senses the presence of a cancer cell. And that's how we use the logic gates, which work in response to inputs like cancerous DNA. Then the output can be the production of a small molecule or the release of a small molecule that can then go and kill what needs killing, in this case, a cancer cell. So we would like to develop applications that use this technology to control the logic gate response of a drug’s delivery to a cell.”
Although platforms like TRUMPET are making progress, a lot more work must be done before they can be used commercially. “The process of translating mechanisms and architecture from biology to computing and vice versa is still an art rather than a science,” says Forrest. “It requires deep computer science and biology knowledge,” she adds. “Some people have compared interdisciplinary science to fusion restaurants—not all combinations are successful, but when they are, the results are remarkable.”
Crickets are low on fat, high on protein, and can be farmed sustainably. They are also crunchy.
In today’s podcast episode, Leaps.org Deputy Editor Lina Zeldovich speaks about the health and ecological benefits of farming crickets for human consumption with Bicky Nguyen, who joins Lina from Vietnam. Bicky and her business partner Nam Dang operate an insect farm named CricketOne. Motivated by the idea of sustainable and healthy protein production, they started their unconventional endeavor a few years ago, despite numerous naysayers who didn’t believe that humans would ever consider munching on bugs.
Yet, making creepy crawlers part of our diet offers many health and planetary advantages. Food production needs to match the rise in global population, estimated to reach 10 billion by 2050. One challenge is that some of our current practices are inefficient, polluting and wasteful. According to nonprofit EarthSave.org, it takes 2,500 gallons of water, 12 pounds of grain, 35 pounds of topsoil and the energy equivalent of one gallon of gasoline to produce one pound of feedlot beef, although exact statistics vary between sources.
Meanwhile, insects are easy to grow, high on protein and low on fat. When roasted with salt, they make crunchy snacks. When chopped up, they transform into delicious pâtes, says Bicky, who invents her own cricket recipes and serves them at industry and public events. Maybe that’s why some research predicts that edible insects market may grow to almost $10 billion by 2030. Tune in for a delectable chat on this alternative and sustainable protein.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
Further reading:
More info on Bicky Nguyen
https://yseali.fulbright.edu.vn/en/faculty/bicky-n...
The environmental footprint of beef production
https://www.earthsave.org/environment.htm
https://www.watercalculator.org/news/articles/beef-king-big-water-footprints/
https://www.frontiersin.org/articles/10.3389/fsufs.2019.00005/full
https://ourworldindata.org/carbon-footprint-food-methane
Insect farming as a source of sustainable protein
https://www.insectgourmet.com/insect-farming-growing-bugs-for-protein/
https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/insect-farming
Cricket flour is taking the world by storm
https://www.cricketflours.com/
https://talk-commerce.com/blog/what-brands-use-cricket-flour-and-why/

Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.