Scientists are working on eye transplants for vision loss. Who will sign up?

Often called the window to the soul, the eyes are more sacred than other body parts, at least for some.
Awash in a fluid finely calibrated to keep it alive, a human eye rests inside a transparent cubic device. This ECaBox, or Eyes in a Care Box, is a one-of-a-kind system built by scientists at Barcelona’s Centre for Genomic Regulation (CRG). Their goal is to preserve human eyes for transplantation and related research.
In recent years, scientists have learned to transplant delicate organs such as the liver, lungs or pancreas, but eyes are another story. Even when preserved at the average transplant temperature of 4 Centigrade, they last for 48 hours max. That's one explanation for why transplanting the whole eye isn’t possible—only the cornea, the dome-shaped, outer layer of the eye, can withstand the procedure. The retina, the layer at the back of the eyeball that turns light into electrical signals, which the brain converts into images, is extremely difficult to transplant because it's packed with nerve tissue and blood vessels.
These challenges also make it tough to research transplantation. “This greatly limits their use for experiments, particularly when it comes to the effectiveness of new drugs and treatments,” said Maria Pia Cosma, a biologist at Barcelona’s Centre for Genomic Regulation (CRG), whose team is working on the ECaBox.
Eye transplants are desperately needed, but they're nowhere in sight. About 12.7 million people worldwide need a corneal transplant, which means that only one in 70 people who require them, get them. The gaps are international. Eye banks in the United Kingdom are around 20 percent below the level needed to supply hospitals, while Indian eye banks, which need at least 250,000 corneas per year, collect only around 45 to 50 thousand donor corneas (and of those 60 to 70 percent are successfully transplanted).
As for retinas, it's impossible currently to put one into the eye of another person. Artificial devices can be implanted to restore the sight of patients suffering from severe retinal diseases, but the number of people around the world with such “bionic eyes” is less than 600, while in America alone 11 million people have some type of retinal disease leading to severe vision loss. Add to this an increasingly aging population, commonly facing various vision impairments, and you have a recipe for heavy burdens on individuals, the economy and society. In the U.S. alone, the total annual economic impact of vision problems was $51.4 billion in 2017.
Even if you try growing tissues in the petri dish route into organoids mimicking the function of the human eye, you will not get the physiological complexity of the structure and metabolism of the real thing, according to Cosma. She is a member of a scientific consortium that includes researchers from major institutions from Spain, the U.K., Portugal, Italy and Israel. The consortium has received about $3.8 million from the European Union to pursue innovative eye research. Her team’s goal is to give hope to at least 2.2 billion people across the world afflicted with a vision impairment and 33 million who go through life with avoidable blindness.
Their method? Resuscitating cadaveric eyes for at least a month.
If we succeed, it will be the first intact human model of the eye capable of exploring and analyzing regenerative processes ex vivo. -- Maria Pia Cosma.
“We proposed to resuscitate eyes, that is to restore the global physiology and function of human explanted tissues,” Cosma said, referring to living tissues extracted from the eye and placed in a medium for culture. Their ECaBox is an ex vivo biological system, in which eyes taken from dead donors are placed in an artificial environment, designed to preserve the eye’s temperature and pH levels, deter blood clots, and remove the metabolic waste and toxins that would otherwise spell their demise.

Scientists work on resuscitating eyes in the lab of Maria Pia Cosma.
Courtesy of Maria Pia Cosma.
“One of the great challenges is the passage of the blood in the capillary branches of the eye, what we call long-term perfusion,” Cosma said. Capillaries are an intricate network of very thin blood vessels that transport blood, nutrients and oxygen to cells in the body’s organs and systems. To maintain the garland-shaped structure of this network, sufficient amounts of oxygen and nutrients must be provided through the eye circulation and microcirculation. “Our ambition is to combine perfusion of the vessels with artificial blood," along with using a synthetic form of vitreous, or the gel-like fluid that lets in light and supports the the eye's round shape, Cosma said.
The scientists use this novel setup with the eye submersed in its medium to keep the organ viable, so they can test retinal function. “If we succeed, we will ensure full functionality of a human organ ex vivo. It will be the first intact human model of the eye capable of exploring and analyzing regenerative processes ex vivo,” Cosma added.
A rapidly developing field of regenerative medicine aims to stimulate the body's natural healing processes and restore or replace damaged tissues and organs. But for people with retinal diseases, regenerative medicine progress has been painfully slow. “Experiments on rodents show progress, but the risks for humans are unacceptable,” Cosma said.
The ECaBox could boost progress with regenerative medicine for people with retinal diseases, which has been painfully slow because human experiments involving their eyes are too risky. “We will test emerging treatments while reducing animal research, and greatly accelerate the discovery and preclinical research phase of new possible treatments for vision loss at significantly reduced costs,” Cosma explained. Much less time and money would be wasted during the drug discovery process. Their work may even make it possible to transplant the entire eyeball for those who need it.
“It is a very exciting project,” said Sanjay Sharma, a professor of ophthalmology and epidemiology at Queen's University, in Kingston, Canada. “The ability to explore and monitor regenerative interventions will increasingly be of importance as we develop therapies that can regenerate ocular tissues, including the retina.”
Seemingly, there's no sacred religious text or a holy book prohibiting the practice of eye donation.
But is the world ready for eye transplants? “People are a bit weird or very emotional about donating their eyes as compared to other organs,” Cosma said. And much can be said about the problem of eye donor shortage. Concerns include disfigurement and healthcare professionals’ fear that the conversation about eye donation will upset the departed person’s relatives because of cultural or religious considerations. As just one example, Sharma noted the paucity of eye donations in his home country, Canada.
Yet, experts like Sharma stress the importance of these donations for both the recipients and their family members. “It allows them some psychological benefit in a very difficult time,” he said. So why are global eye banks suffering? Is it because the eyes are the windows to the soul?
Seemingly, there's no sacred religious text or a holy book prohibiting the practice of eye donation. In fact, most major religions of the world permit and support organ transplantation and donation, and by extension eye donation, because they unequivocally see it as an “act of neighborly love and charity.” In Hinduism, the concept of eye donation aligns with the Hindu principle of daan or selfless giving, where individuals donate their organs or body after death to benefit others and contribute to society. In Islam, eye donation is a form of sadaqah jariyah, a perpetual charity, as it can continue to benefit others even after the donor's death.
Meanwhile, Buddhist masters teach that donating an organ gives another person the chance to live longer and practice dharma, the universal law and order, more meaningfully; they also dismiss misunderstandings of the type “if you donate an eye, you’ll be born without an eye in the next birth.” And Christian teachings emphasize the values of love, compassion, and selflessness, all compatible with organ donation, eye donation notwithstanding; besides, those that will have a house in heaven, will get a whole new body without imperfections and limitations.
The explanation for people’s resistance may lie in what Deepak Sarma, a professor of Indian religions and philosophy at Case Western Reserve University in Cleveland, calls “street interpretation” of religious or spiritual dogmas. Consider the mechanism of karma, which is about the causal relation between previous and current actions. “Maybe some Hindus believe there is karma in the eyes and, if the eye gets transplanted into another person, they will have to have that karmic card from now on,” Sarma said. “Even if there is peculiar karma due to an untimely death–which might be interpreted by some as bad karma–then you have the karma of the recipient, which is tremendously good karma, because they have access to these body parts, a tremendous gift,” Sarma said. The overall accumulation is that of good karma: “It’s a beautiful kind of balance,” Sarma said.
For the Jews, Christians, and Muslims who believe in the physical resurrection of the body that will be made new in an afterlife, the already existing body is sacred since it will be the basis of a new refashioned body in an afterlife.---Omar Sultan Haque.
With that said, Sarma believes it is a fallacy to personify or anthropomorphize the eye, which doesn’t have a soul, and stresses that the karma attaches itself to the soul and not the body parts. But for scholars like Omar Sultan Haque—a psychiatrist and social scientist at Harvard Medical School, investigating questions across global health, anthropology, social psychology, and bioethics—the hierarchy of sacredness of body parts is entrenched in human psychology. You cannot equate the pinky toe with the face, he explained.
“The eyes are the window to the soul,” Haque said. “People have a hierarchy of body parts that are considered more sacred or essential to the self or soul, such as the eyes, face, and brain.” In his view, the techno-utopian transhumanist communities (especially those in Silicon Valley) have reduced the totality of a person to a mere material object, a “wet robot” that knows no sacredness or hierarchy of human body parts. “But for the Jews, Christians, and Muslims who believe in the physical resurrection of the body that will be made new in an afterlife, the [already existing] body is sacred since it will be the basis of a new refashioned body in an afterlife,” Haque said. “You cannot treat the body like any old material artifact, or old chair or ragged cloth, just because materialistic, secular ideologies want so,” he continued.
For Cosma and her peers, however, the very definition of what is alive or not is a bit semantic. “As soon as we die, the electrophysiological activity in the eye stops,” she said. “The goal of the project is to restore this activity as soon as possible before the highly complex tissue of the eye starts degrading.” Cosma’s group doesn’t yet know when they will be able to keep the eyes alive and well in the ECaBox, but the consensus is that the sooner the better. Hopefully, the taboos and fears around the eye donations will dissipate around the same time.
DNA- and RNA-based electronic implants may revolutionize healthcare
The test tubes contain tiny DNA/enzyme-based circuits, which comprise TRUMPET, a new type of electronic device, smaller than a cell.
Implantable electronic devices can significantly improve patients’ quality of life. A pacemaker can encourage the heart to beat more regularly. A neural implant, usually placed at the back of the skull, can help brain function and encourage higher neural activity. Current research on neural implants finds them helpful to patients with Parkinson’s disease, vision loss, hearing loss, and other nerve damage problems. Several of these implants, such as Elon Musk’s Neuralink, have already been approved by the FDA for human use.
Yet, pacemakers, neural implants, and other such electronic devices are not without problems. They require constant electricity, limited through batteries that need replacements. They also cause scarring. “The problem with doing this with electronics is that scar tissue forms,” explains Kate Adamala, an assistant professor of cell biology at the University of Minnesota Twin Cities. “Anytime you have something hard interacting with something soft [like muscle, skin, or tissue], the soft thing will scar. That's why there are no long-term neural implants right now.” To overcome these challenges, scientists are turning to biocomputing processes that use organic materials like DNA and RNA. Other promised benefits include “diagnostics and possibly therapeutic action, operating as nanorobots in living organisms,” writes Evgeny Katz, a professor of bioelectronics at Clarkson University, in his book DNA- And RNA-Based Computing Systems.
While a computer gives these inputs in binary code or "bits," such as a 0 or 1, biocomputing uses DNA strands as inputs, whether double or single-stranded, and often uses fluorescent RNA as an output.
Adamala’s research focuses on developing such biocomputing systems using DNA, RNA, proteins, and lipids. Using these molecules in the biocomputing systems allows the latter to be biocompatible with the human body, resulting in a natural healing process. In a recent Nature Communications study, Adamala and her team created a new biocomputing platform called TRUMPET (Transcriptional RNA Universal Multi-Purpose GatE PlaTform) which acts like a DNA-powered computer chip. “These biological systems can heal if you design them correctly,” adds Adamala. “So you can imagine a computer that will eventually heal itself.”
The basics of biocomputing
Biocomputing and regular computing have many similarities. Like regular computing, biocomputing works by running information through a series of gates, usually logic gates. A logic gate works as a fork in the road for an electronic circuit. The input will travel one way or another, giving two different outputs. An example logic gate is the AND gate, which has two inputs (A and B) and two different results. If both A and B are 1, the AND gate output will be 1. If only A is 1 and B is 0, the output will be 0 and vice versa. If both A and B are 0, the result will be 0. While a computer gives these inputs in binary code or "bits," such as a 0 or 1, biocomputing uses DNA strands as inputs, whether double or single-stranded, and often uses fluorescent RNA as an output. In this case, the DNA enters the logic gate as a single or double strand.
If the DNA is double-stranded, the system “digests” the DNA or destroys it, which results in non-fluorescence or “0” output. Conversely, if the DNA is single-stranded, it won’t be digested and instead will be copied by several enzymes in the biocomputing system, resulting in fluorescent RNA or a “1” output. And the output for this type of binary system can be expanded beyond fluorescence or not. For example, a “1” output might be the production of the enzyme insulin, while a “0” may be that no insulin is produced. “This kind of synergy between biology and computation is the essence of biocomputing,” says Stephanie Forrest, a professor and the director of the Biodesign Center for Biocomputing, Security and Society at Arizona State University.
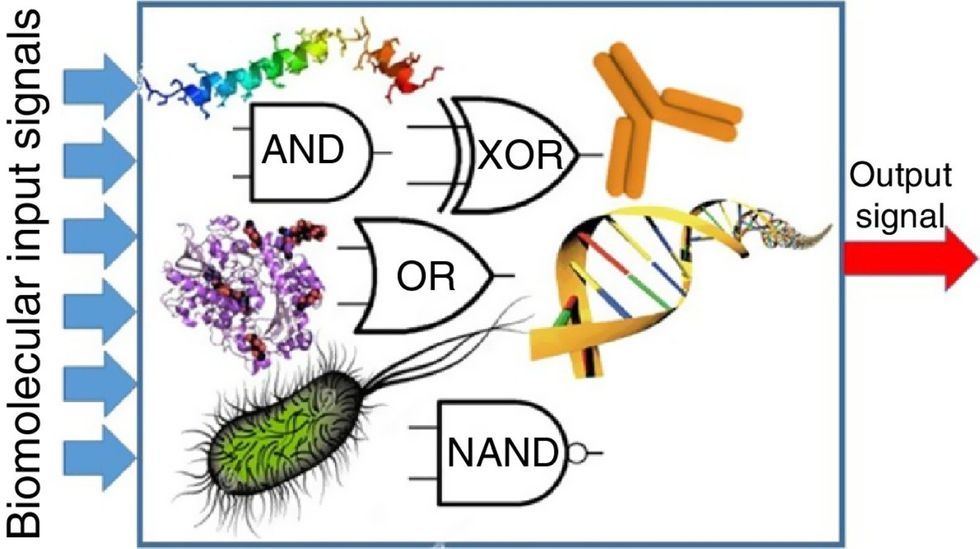
Biocomputing circles are made of DNA, RNA, proteins and even bacteria.
Evgeny Katz
The TRUMPET’s promise
Depending on whether the biocomputing system is placed directly inside a cell within the human body, or run in a test-tube, different environmental factors play a role. When an output is produced inside a cell, the cell's natural processes can amplify this output (for example, a specific protein or DNA strand), creating a solid signal. However, these cells can also be very leaky. “You want the cells to do the thing you ask them to do before they finish whatever their businesses, which is to grow, replicate, metabolize,” Adamala explains. “However, often the gate may be triggered without the right inputs, creating a false positive signal. So that's why natural logic gates are often leaky." While biocomputing outside a cell in a test tube can allow for tighter control over the logic gates, the outputs or signals cannot be amplified by a cell and are less potent.
TRUMPET, which is smaller than a cell, taps into both cellular and non-cellular biocomputing benefits. “At its core, it is a nonliving logic gate system,” Adamala states, “It's a DNA-based logic gate system. But because we use enzymes, and the readout is enzymatic [where an enzyme replicates the fluorescent RNA], we end up with signal amplification." This readout means that the output from the TRUMPET system, a fluorescent RNA strand, can be replicated by nearby enzymes in the platform, making the light signal stronger. "So it combines the best of both worlds,” Adamala adds.
These organic-based systems could detect cancer cells or low insulin levels inside a patient’s body.
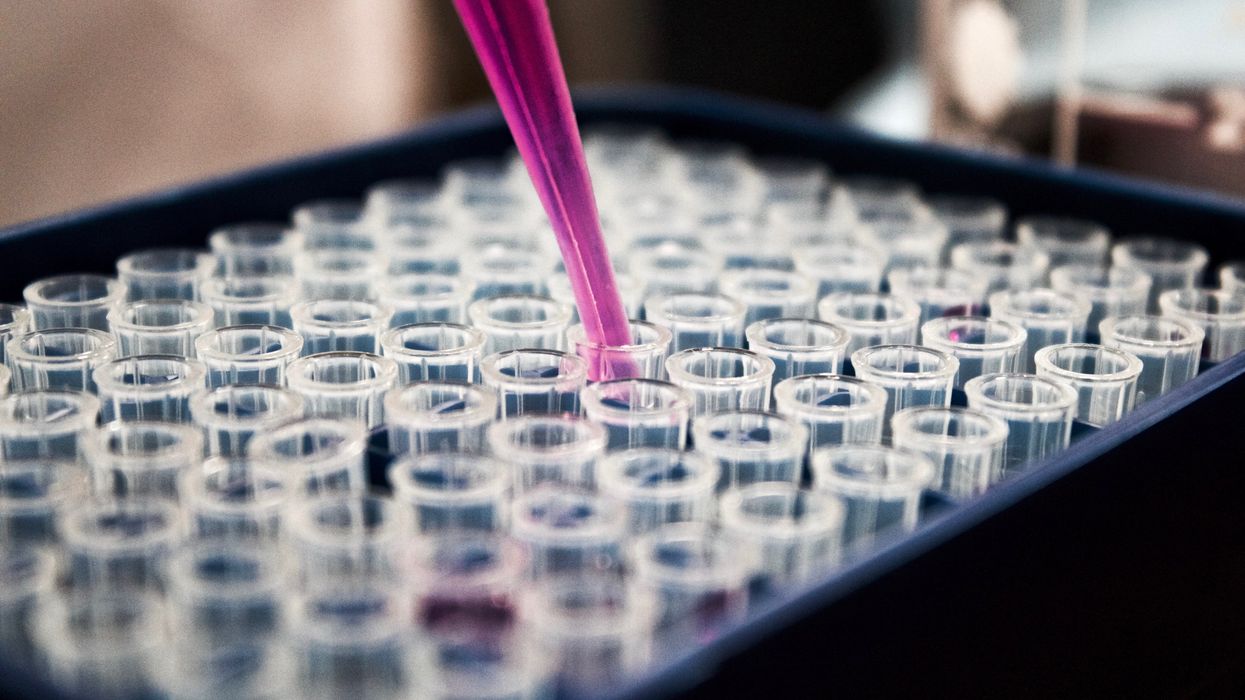
The TRUMPET biocomputing process is relatively straightforward. “If the DNA [input] shows up as single-stranded, it will not be digested [by the logic gate], and you get this nice fluorescent output as the RNA is made from the single-stranded DNA, and that's a 1,” Adamala explains. "And if the DNA input is double-stranded, it gets digested by the enzymes in the logic gate, and there is no RNA created from the DNA, so there is no fluorescence, and the output is 0." On the story's leading image above, if the tube is "lit" with a purple color, that is a binary 1 signal for computing. If it's "off" it is a 0.
While still in research, TRUMPET and other biocomputing systems promise significant benefits to personalized healthcare and medicine. These organic-based systems could detect cancer cells or low insulin levels inside a patient’s body. The study’s lead author and graduate student Judee Sharon is already beginning to research TRUMPET's ability for earlier cancer diagnoses. Because the inputs for TRUMPET are single or double-stranded DNA, any mutated or cancerous DNA could theoretically be detected from the platform through the biocomputing process. Theoretically, devices like TRUMPET could be used to detect cancer and other diseases earlier.
Adamala sees TRUMPET not only as a detection system but also as a potential cancer drug delivery system. “Ideally, you would like the drug only to turn on when it senses the presence of a cancer cell. And that's how we use the logic gates, which work in response to inputs like cancerous DNA. Then the output can be the production of a small molecule or the release of a small molecule that can then go and kill what needs killing, in this case, a cancer cell. So we would like to develop applications that use this technology to control the logic gate response of a drug’s delivery to a cell.”
Although platforms like TRUMPET are making progress, a lot more work must be done before they can be used commercially. “The process of translating mechanisms and architecture from biology to computing and vice versa is still an art rather than a science,” says Forrest. “It requires deep computer science and biology knowledge,” she adds. “Some people have compared interdisciplinary science to fusion restaurants—not all combinations are successful, but when they are, the results are remarkable.”
Crickets are low on fat, high on protein, and can be farmed sustainably. They are also crunchy.
In today’s podcast episode, Leaps.org Deputy Editor Lina Zeldovich speaks about the health and ecological benefits of farming crickets for human consumption with Bicky Nguyen, who joins Lina from Vietnam. Bicky and her business partner Nam Dang operate an insect farm named CricketOne. Motivated by the idea of sustainable and healthy protein production, they started their unconventional endeavor a few years ago, despite numerous naysayers who didn’t believe that humans would ever consider munching on bugs.
Yet, making creepy crawlers part of our diet offers many health and planetary advantages. Food production needs to match the rise in global population, estimated to reach 10 billion by 2050. One challenge is that some of our current practices are inefficient, polluting and wasteful. According to nonprofit EarthSave.org, it takes 2,500 gallons of water, 12 pounds of grain, 35 pounds of topsoil and the energy equivalent of one gallon of gasoline to produce one pound of feedlot beef, although exact statistics vary between sources.
Meanwhile, insects are easy to grow, high on protein and low on fat. When roasted with salt, they make crunchy snacks. When chopped up, they transform into delicious pâtes, says Bicky, who invents her own cricket recipes and serves them at industry and public events. Maybe that’s why some research predicts that edible insects market may grow to almost $10 billion by 2030. Tune in for a delectable chat on this alternative and sustainable protein.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
Further reading:
More info on Bicky Nguyen
https://yseali.fulbright.edu.vn/en/faculty/bicky-n...
The environmental footprint of beef production
https://www.earthsave.org/environment.htm
https://www.watercalculator.org/news/articles/beef-king-big-water-footprints/
https://www.frontiersin.org/articles/10.3389/fsufs.2019.00005/full
https://ourworldindata.org/carbon-footprint-food-methane
Insect farming as a source of sustainable protein
https://www.insectgourmet.com/insect-farming-growing-bugs-for-protein/
https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/insect-farming
Cricket flour is taking the world by storm
https://www.cricketflours.com/
https://talk-commerce.com/blog/what-brands-use-cricket-flour-and-why/

Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.