A brownie cake and whipped cream beckon on a plate.
Imagine eating a slice of cake for breakfast. It's deliciously indulgent, but instead of your blood sugar spiking, your body processes all that sweetness as a healthy high-protein meal. It may sound like sci-fi, but this scenario is not necessarily far off.
"People with diabetes could especially benefit because sweet proteins don't trigger a need for insulin."
The Lowdown
An award-winning agtech startup called Amai is developing "sweet proteins," based on the molecular structure of naturally occuring exotic fruits. These new sugar substitutes could potentially replace artificial sweeteners and help people who are trying to curb their sugar intake. People with diabetes could especially benefit because sweet proteins don't trigger a need for insulin.
While there is a sweet protein currently on the market today called thaumatin, it's expensive, has a short shelf life, and is lacking in the taste department. But Amai's proteins taste 70 to 100 percent identical to the sweet ones found in nature. Once their molecular structure is designed through a sophisticated computing platform, they are made through fermentation, which is akin to brewing beer. These non-GMO proteins are over 10,000 times sweeter than sugar, which means much less needs to be produced and used.
Diseases like diabetes and heart disease, which are often linked to sugar overconsumption, have been on a major upswing over the last few decades, especially in the United States. According to the CDC, 100 million adults in the United States are now living with diabetes or prediabetes, which if not treated, often leads to type 2 diabetes within five years. By 2030, scientists predict cases of diabetes in the U.S. will increase by 54 percent. If sugar proteins like the type Amai is creating become widely available, these numbers could begin to decrease.
Next Up
Amai's sweet proteins are still in the research and development stage, but the Israeli startup is raising significant funding that should help expedite the process. They're also substantially upping their production ability by expanding their facilities.
Will consumers be comfortable ingesting a lab-designed food product?
And in March, the USDA and FDA announced plans to regulate cell-cultured foods, the category in which these sugar proteins would fall, so Amai researchers are hopeful they'll have an easier path to approval once their product is market ready.
Open Questions
All this progress may sound promising, but Amai still has a long way to go before the reality of healthy cake becomes tangible. Some questions to consider: Will consumers be comfortable ingesting a lab-designed food product? Will it taste enough like real sugar?
And if some products and brands begin to adopt it, will it ever overtake the real thing in popularity and make a dent in diseases like diabetes and obesity? Only time, more research, and a lot more money will tell, but in the meantime, feel free to daydream about eating entire pints of ice cream without needing to hit the gym.
Can You Trust Your Gut for Food Advice?
Which foods are actually healthy for your individual gut microbiome? Several companies are offering personalized dietary guidance based on your test results, but their answers in one experiment turned up with some conflicting advice.
I recently got on the scale to weigh myself, thinking I've got to eat better. With so many trendy diets today claiming to improve health, from Keto to Paleo to Whole30, it can be confusing to figure out what we should and shouldn't eat for optimal nutrition.
A number of companies are now selling the concept of "personalized" nutrition based on the genetic makeup of your individual gut bugs.
My next thought was: I've got to lose a few pounds.
Consider a weird factoid: In addition to my fat, skin, bone and muscle, I'm carrying around two or three pounds of straight-up bacteria. Like you, I am the host to trillions of micro-organisms that live in my gut and are collectively known as my microbiome. An explosion of research has occurred in the last decade to try to understand exactly how these microbial populations, which are unique to each of us, may influence our overall health and potentially even our brains and behavior.
Lots of mysteries still remain, but it is established that these "bugs" are crucial to keeping our body running smoothly, performing functions like stimulating the immune system, synthesizing important vitamins, and aiding digestion. The field of microbiome science is evolving rapidly, and a number of companies are now selling the concept of "personalized" nutrition based on the genetic makeup of your individual gut bugs. The two leading players are Viome and DayTwo, but the landscape includes the newly launched startup Onegevity Health and others like Thryve, which offers customized probiotic supplements in addition to dietary recommendations.
The idea has immediate appeal – if science could tell you exactly what to make for lunch and what to avoid, you could forget about the fad diets and go with your own bespoke food pyramid. Wondering if the promise might be too good to be true, I decided to perform my own experiment.
Last fall, I sent the identical fecal sample to both Viome (I paid $425, but the price has since dropped to $299) and DayTwo ($349). A couple of months later, both reports finally arrived, and I eagerly opened each app to compare their recommendations.
First, I examined my results from Viome, which was founded in 2016 in Cupertino, Calif., and declares without irony on its website that "conflicting food advice is now obsolete."
I learned I have "average" metabolic fitness and "average" inflammatory activity in my gut, which are scores that the company defines based on a proprietary algorithm. But I have "low" microbial richness, with only 62 active species of bacteria identified in my sample, compared with the mean of 157 in their test population. I also received a list of the specific species in my gut, with names like Lactococcus and Romboutsia.
But none of it meant anything to me without actionable food advice, so I clicked through to the Recommendations page and found a list of My Superfoods (cranberry, garlic, kale, salmon, turmeric, watermelon, and bone broth) and My Foods to Avoid (chickpeas, kombucha, lentils, and rice noodles). There was also a searchable database of many foods that had been categorized for me, like "bell pepper; minimize" and "beef; enjoy."
"I just don't think sufficient data is yet available to make reliable personalized dietary recommendations based on one's microbiome."
Next, I looked at my results from DayTwo, which was founded in 2015 from research out of the Weizmann Institute of Science in Israel, and whose pitch to consumers is, "Blood sugar made easy. The algorithm diet personalized to you."
This app had some notable differences. There was no result about my metabolic fitness, microbial richness, or list of the species in my sample. There was also no list of superfoods or foods to avoid. Instead, the app encouraged me to build a meal by searching for foods in their database and combining them in beneficial ways for my blood sugar. Two slices of whole wheat bread received a score of 2.7 out of 10 ("Avoid"), but if combined with one cup of large curd cottage cheese, the score improved to 6.8 ("Limit"), and if I added two hard-boiled eggs, the score went up to 7.5 ("Good").
Perusing my list of foods with "Excellent" scores, I noticed some troubling conflicts with the other app. Lentils, which had been a no-no according to Viome, received high marks from DayTwo. Ditto for Kombucha. My purported superfood of cranberry received low marks. Almonds got an almost perfect score (9.7) while Viome told me to minimize them. I found similarly contradictory advice for foods I regularly eat, including navel oranges, peanuts, pork, and beets.
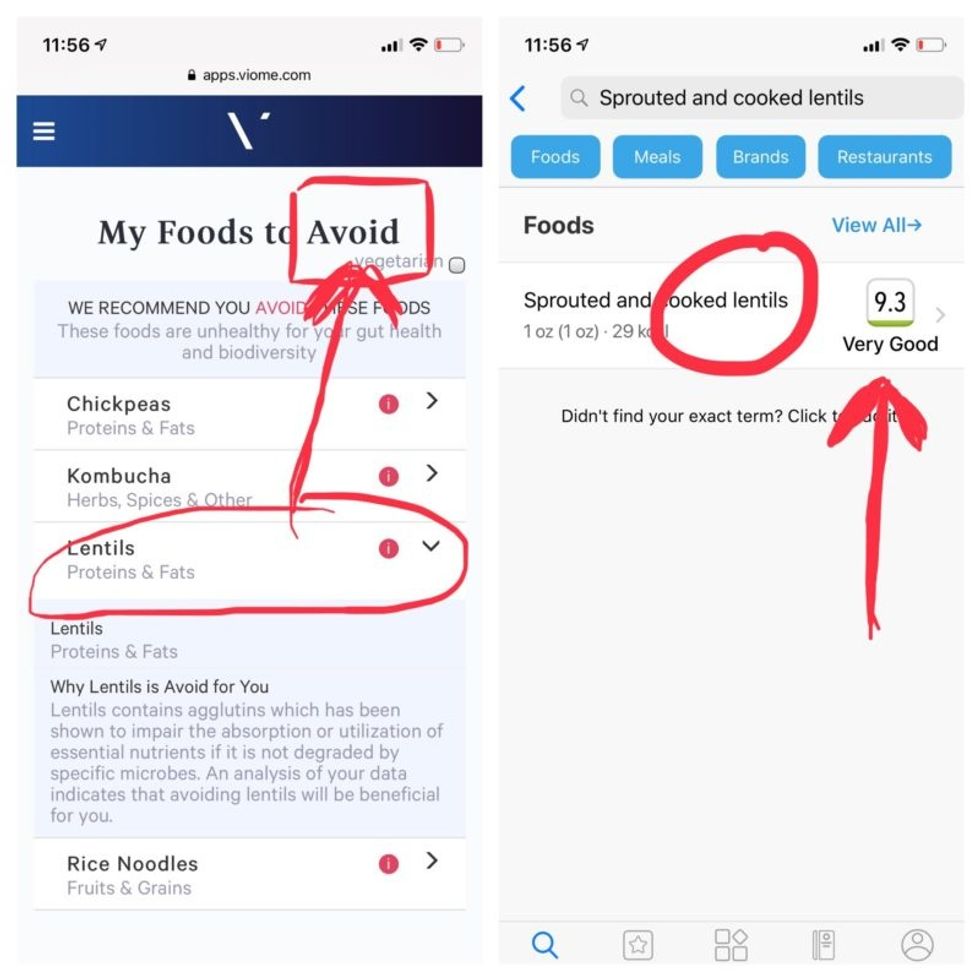
Contradictory dietary guidance that Kira Peikoff received from Viome (left) and DayTwo from an identical sample.
To be sure, there was some overlap. Both apps agreed on rice noodles (bad), chickpeas (bad), honey (bad), carrots (good), and avocado (good), among other foods.
But still, I was left scratching my head. Which set of recommendations should I trust, if either? And what did my results mean for the accuracy of this nascent field?
I called a couple of experts to find out.
"I have worked on the microbiome and nutrition for the last 20 years and I would be absolutely incapable of finding you evidence in the scientific literature that lentils have a detrimental effect based on the microbiome," said Dr. Jens Walter, an Associate Professor and chair for Nutrition, Microbes, and Gastrointestinal Health at the University of Alberta. "I just don't think sufficient data is yet available to make reliable personalized dietary recommendations based on one's microbiome. And even if they would have proprietary algorithms, at least one of them is not doing it right."
There is definite potential for personalized nutrition based on the microbiome, he said, but first, predictive models must be built and standardized, then linked to clinical endpoints, and tested in a large sample of healthy volunteers in order to enable extrapolations for the general population.
"It is mindboggling what you would need to do to make this work," he observed. "There are probably hundreds of relevant dietary compounds, then the microbiome has at least a hundred relevant species with a hundred or more relevant genes each, then you'd have to put all this together with relevant clinical outcomes. And there's a hundred-fold variation in that information between individuals."
However, Walter did acknowledge that the companies might be basing their algorithms on proprietary data that could potentially connect all the dots. I reached out to them to find out.
Amir Golan, the Chief Commercial Officer of DayTwo, told me, "It's important to emphasize this is a prediction, as the microbiome field is in a very early stage of research." But he added, "I believe we are the only company that has very solid science published in top journals and we can bring very actionable evidence and benefit to our uses."
He was referring to pioneering work out of the Weizmann Institute that was published in 2015 in the journal Cell, which logged the glycemic responses of 800 people in response to nearly 50,000 meals; adding information about the subjects' microbiomes enabled more accurate glycemic response predictions. Since then, Golan said, additional trials have been conducted, most recently with the Mayo Clinic, to duplicate the results, and other studies are ongoing whose results have not yet been published.
He also pointed out that the microbiome was merely one component that goes into building a client's profile, in addition to medical records, including blood glucose levels. (I provided my HbA1c levels, a measure of average blood sugar over the previous several months.)
"We are not saying we want to improve your gut microbiome. We provide a dynamic tool to help guide what you should eat to control your blood sugar and think about combinations," he said. "If you eat one thing, or with another, it will affect you in a different way."
Viome acknowledged that the two companies are taking very different approaches.
"DayTwo is primarily focused on the glycemic response," Naveen Jain, the CEO, told me. "If you can only eat butter for rest of your life, you will have no glycemic response but will probably die of a heart attack." He laughed. "Whereas we came from very different angle – what is happening inside the gut at a microbial level? When you eat food like spinach, how will that be metabolized in the gut? Will it produce the nutrients you need or cause inflammation?"
He said his team studied 1000 people who were on continuous glucose monitoring and fed them 45,000 meals, then built a proprietary data prediction model, looking at which microbes existed and how they actively broke down the food.
Jain pointed out that DayTwo sequences the DNA of the microbes, while Viome sequences the RNA – the active expression of DNA. That difference, in his opinion, is key to making accurate predictions.
"DNA is extremely stable, so when you eat any food and measure the DNA [in a fecal sample], you get all these false positives--you get DNA from plant food and meat, and you have no idea if those organisms are dead and simply transient, or actually exist. With RNA, you see what is actually alive in the gut."
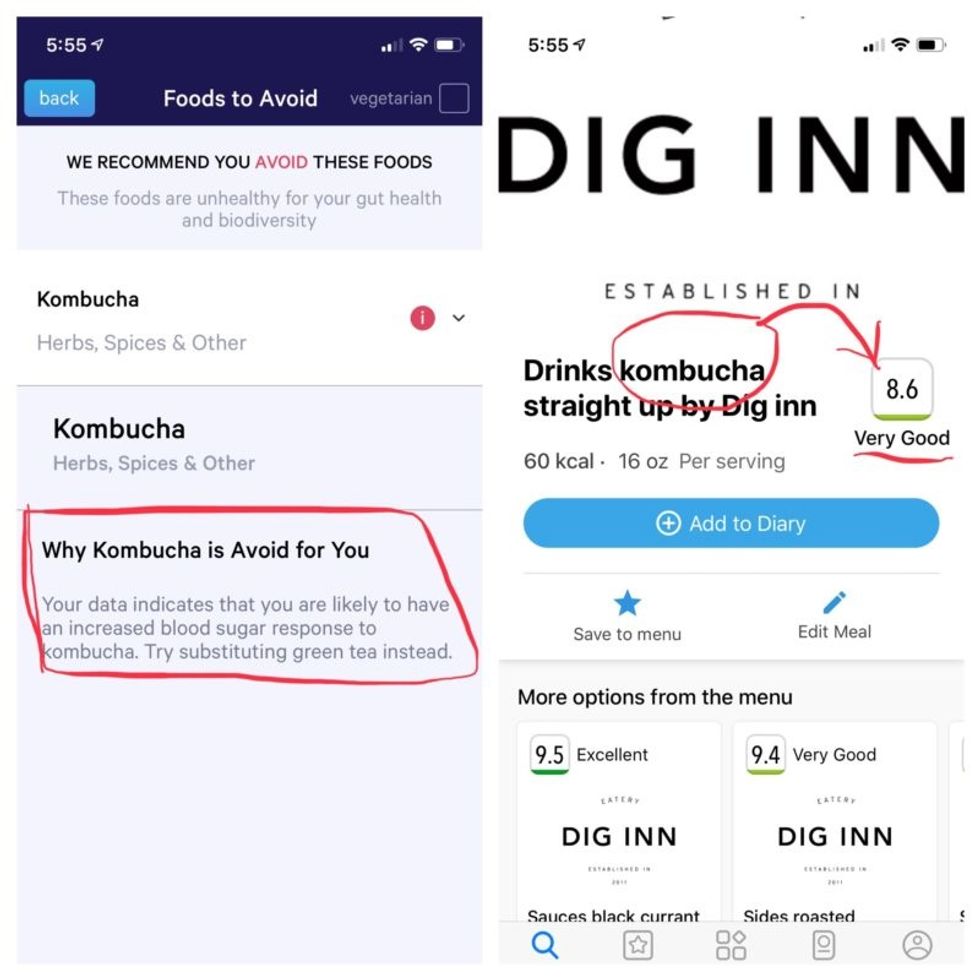
More contradictory food advice from Viome (left) and DayTwo.
Note that controversy exists over how it is possible with a fecal sample to effectively measure RNA, which degrades within minutes, though Jain said that his company has the technology to keep RNA stable for fourteen days.
Viome's approach, Jain maintains, is 90 percent accurate, based on as-yet unpublished data; a patent was filed just last week. DayTwo's approach is 66 percent accurate according to the latest published research.
Natasha Haskey, a registered dietician and doctoral student conducting research in the field of microbiome science and nutrition, is skeptical of both companies. "We can make broad statements, like eat more fruits and vegetables and fiber, but when it comes to specific foods, the science is just not there yet," she said. "I think there is a future, and we will be doing that someday, but not yet. Maybe we will be closer in ten years."
Professor Walter wholeheartedly agrees with Haskey, and suggested that if people want to eat a gut-healthy diet, they should focus on beneficial oils, fruits and vegetables, fish, a variety of whole grains, poultry and beans, and limit red meat and cheese, as well as avoid processed meats.
"These services are far over the tips of their science skis," Arthur Caplan, the founding head of New York University's Division of Medical Ethics, said in an email. "We simply don't know enough about the gut microbiome, its fluctuations and variability from person to person to support general [direct-to-consumer] testing. This is simply premature. We need standards for accuracy, specificity, and sensitivity, plus mandatory competent counseling for all such testing. They don't exist. Neither should DTC testing—yet."
Meanwhile, it's time for lunch. I close out my Viome and DayTwo apps and head to the kitchen to prepare a peanut butter sandwich. My gut tells me I'll be just fine.
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
Wild-Caught Seafood Has Been Notoriously Shady – Until Now
The Dock to Dish model has expanded across North and Central America. Above, artisanal fishermen on the Pacific coast of Costa Rica head out to sea seeking schools of abundant forage fish to supply the program.
In 2012, entrepreneur Sean Barrett founded Dock to Dish in Montauk, New York. It connected local fishermen and women with local chefs, enabling the chefs to serve hyper-fresh seafood – with the caveat that they didn't know what would be on their menus until it arrived in their kitchens the night before.
"Since we're not a seafood-centric culture, people don't know what's what, where fish are from, and when they're in season, making them easy to dupe."
In June of 2017, The United Nations Foundation designated Dock to Dish as one of the top breakthrough innovations that can scale to solve the ocean's grand challenges. His company has since expanded across the Americas and has just opened up shop in Fiji. Leapsmag recently chatted with Barrett about his inspirations and ideas for how to overcome the hurdles of farming wild seafood. This interview has been edited and condensed for clarity.
What inspired you to start Dock to Dish?
The short story is "A Tale of Two Hills."
The first is Quail Hill Farm in Amagansett. I grew up in the commercial fishing port of Chinicock in the 1980's and 90's, working on my family's dock from an early age and in the restaurant industry in my teens. By my thirties, I had accrued my 10,000 hours of experience in both dock and dish. I watched the food system shift from local to global, especially in seafood. By the early 2000's, over 90 percent of seafood in the U.S. was imported. It was bad.
Quail Hill was the first CSA [Community Supported Agriculture, in which customers pay up front for a share in whatever crops grow (or don't) on the farm that season] in the U.S., founded in 1990. So people in the area were accustomed to getting their produce that way. Scott Chaskey, the poet farmer at Quail Hill, really helped crystallize the philosophy for me and inspired me to apply it to seafood. Fishermen had always been bringing a share of their day's catch to their neighbors; now we were just doing it in a more formalized way.
The second is Blue Hill at Stone Barns. [Executive chef and co-owner] Dan Barber literally trademarked the phrase "Know Thy Farmer"; we just expanded it to Know Thy Fisherman and it took off like a rocket ship. His connections in the restaurant world were also indispensable.

17th generation Montauk fisherman Captain Bruce Beckwith (above left) with crew Charlie Etzel (Center) and Jeremy Gould (right).
Do you have any issues that are unique to seafood that a CSA or meat co-op wouldn't face?
This food is WILD. People are totally disconnected from what that word means, and it makes seafood different from everything else. Everything changes when viewed through the prism of that word.
This is the last wild food we eat. It is unpredictable, and subject to variables ranging from currents and tides to which way the wind is blowing. But it is what makes our model so much more impactful and beneficial than the industrialized, demand-driven marketplace that surrounds us. The ocean and its ecosystem are the boss, not chefs and consumers.
There has a been a lot of press about seafood being mislabeled. How and why does that happen? Can Dock to Dish fix it?
Imported, farmed seafood is cheap. Wild, sustainable seafood is not. People are buying low and selling high to make a buck; and while fisheries are extraordinarily regulated, the marketplace isn't. There is no punishment for mislabeling, and no means to correct it. Since we're not a seafood-centric culture, people don't know what's what, where fish are from, and when they're in season, making them easy to dupe. But technology is poised to fix that; DNA testing can test what a fish sample is and where it's from, and SciO handheld spectrometers – soon to be incorporated into smartphones – can analyze the molecular makeup of anything on your plate.
We've created the first ever live tracking system and database for wild fisheries. It is similar to the electronic system used to monitor commercial fisheries, thanks to which the resurgence of wild seafood in U.S. waters is a model for the rest of the world. We have vessel tracking devices on our fishing boats and delivery vans, so the path of each fish is publicly available in real time.

In 2017, Dock to Dish launched the world's first live "end-to-end" tracking system for wild seafood, which provides full chain transparency and next-generation traceability for members.
People are increasingly looking to seafood as a healthier, possibly more sustainable protein option than meat. Can Dock to Dish scale up to accommodate this potentially growing market?
Nope. We can't scale; the supply is finite. That's why the price keeps going up. To avoid becoming "fish for the rich" we are working closely with Greenwave.org to create a network of 3D restorative ocean farms growing kelp and shellfish, which sequester carbon and nitrogen out of the air and soil. Restorative, because sustainable is no longer an option. In fifty years, a plate of seafood will be mostly ocean vegetables with a small amount of finfish as a garnish.



