How Should Genetic Engineering Shape Our Future?

An artist's rendering of genetic codes.
Terror. Error. Success. These are the three outcomes that ethicists evaluating a new technology should fear. The possibility that a breakthrough might be used maliciously. The possibility that newly empowered scientists might make a catastrophic mistake. And the possibility that a technology will be so successful that it will change how we live in ways that we can only guess—and that we may not want.
These tools will allow scientists to practice genetic engineering on a scale that is simultaneously far more precise and far more ambitious than ever before.
It was true for the scientists behind the Manhattan Project, who bequeathed a fear of nuclear terror and nuclear error, even as global security is ultimately defined by these weapons of mass destruction. It was true for the developers of the automobile, whose invention has been weaponized by terrorists and kills 3,400 people by accident each day, even as the more than 1 billion cars on the road today have utterly reshaped where we live and how we move. And it is true for the researchers behind the revolution in gene editing and writing.
Put simply, these tools will allow scientists to practice genetic engineering on a scale that is simultaneously far more precise and far more ambitious than ever before. Editing techniques like CRISPR enable exact genetic repairs through a simple cut and paste of DNA, while synthetic biologists aim to redo entire genomes through the writing and substitution of synthetic genes. The technologies are complementary, and they herald an era when the book of life will be not just readable, but rewritable. Food crops, endangered animals, even the human body itself—all will eventually be programmable.
The benefits are easy to imagine: more sustainable crops; cures for terminal genetic disorders; even an end to infertility. Also easy to picture are the ethical pitfalls as the negative images of those same benefits.
Terror is the most straightforward. States have sought to use biology as a weapon at least since invading armies flung the corpses of plague victims into besieged castles. The 1975 biological weapons convention banned—with general success—the research and production of offensive bioweapons, though a handful of lone terrorists and groups like the Oregon-based Rajneeshee cult have still carried out limited bioweapon attacks. Those incidents ultimately caused little death and damage, in part because medical science is mostly capable of defending us from those pathogens that are most easily weaponized. But gene editing and writing offers the chance to engineer germs that could be far more effective than anything nature could develop. Imagine a virus that combines the lethality of Ebola with the transmissibility of the common cold—and in the new world of biology, if you can imagine something, you will eventually be able to create it.
The benefits are easy to imagine: more sustainable crops; cures for terminal genetic disorders; even an end to infertility. Also easy to picture are the ethical pitfalls.
That's one reason why James Clapper, then the U.S. director of national intelligence, added gene editing to the list of threats posed by "weapons of mass destruction and proliferation" in 2016. But these new tools aren't merely dangerous in the wrong hands—they can also be dangerous in the right hands. The list of labs accidents involving lethal bugs is much longer than you'd want to know, at least if you're the sort of person who likes to sleep at night. The U.S. recently lifted a ban on research that works to make existing pathogens, like the H5N1 avian flu virus, more virulent and transmissible, often using new technologies like gene editing. Such work can help medicine better prepare for what nature might throw at us, but it could also make the consequences of a lab error far more catastrophic. There's also the possibility that the use of gene editing and writing in nature—say, by CRISPRing disease-carrying mosquitoes to make them sterile—could backfire in some unforeseen way. Add in the fact that the techniques behind gene editing and writing are becoming simpler and more automated with every year, and eventually millions of people will be capable—through terror or error—of unleashing something awful on the world.
The good news is that both the government and the researchers driving these technologies are increasingly aware of the risks of bioterror and error. One government program, the Functional Genomic and Computational Assessment of Threats (Fun GCAT), provides funding for scientists to scan genetic data looking for the "accidental or intentional creation of a biological threat." Those in the biotech industry know to keep an eye out for suspicious orders—say, a new customer who orders part of the sequence of the Ebola or smallpox virus. "With every invention there is a good use and a bad use," Emily Leproust, the CEO of the commercial DNA synthesis startup Twist Bioscience, said in a recent interview. "What we try hard to do is put in place as many systems as we can to maximize the good stuff, and minimize any negative impact."
But the greatest ethical challenges in gene editing and writing will arise not from malevolence or mistakes, but from success. Through a new technology called in vitro gametogenesis (IVG), scientists are learning how to turn adult human cells like a piece of skin into lab-made sperm and egg cells. That would be a huge breakthrough for the infertile, or for same-sex couples who want to conceive a child biologically related to both partners. It would also open the door to using gene editing to tinker with those lab-made embryos. At first interventions would address any obvious genetic disorders, but those same tools would likely allow the engineering of a child's intelligence, height and other characteristics. We might be morally repelled today by such an ability, as many scientists and ethicists were repelled by in-vitro fertilization (IVF) when it was introduced four decades ago. Yet more than a million babies in the U.S. have been born through IVF in the years since. Ethics can evolve along with technology.
These new technologies offer control over the code of life, but only we as a society can seize control over where these tools will take us.
Fertility is just one human institution that stands to be changed utterly by gene editing and writing, and it's a change we can at least imagine. As the new biology grows more ambitious, it will alter society in ways we can't begin to picture. Harvard's George Church and New York University's Jef Boeke are leading an effort called HGP-Write to create a completely synthetic human genome. While gene editing allows scientists to make small changes to the genome, the gene synthesis that Church and his collaborators are developing allows for total genetic rewrites. "It's a difference between editing a book and writing one," Church said in an interview earlier this year.
Church is already working on synthesizing organs that would be resistant to viruses, while other researchers like Harris Wang at Columbia University are experimenting with bioengineering mammalian cells to produce nutrients like amino acids that we currently need to get from food. The horizon is endless—and so are the ethical concerns of success. What if parents feel pressure to engineer their children just so they don't fall behind their IVG peers? What if only the rich are able to access synthetic biology technologies that could make them stronger, smarter and longer lived? Could inequality become encoded in the genome?
These are questions that are different from the terror and errors fears around biosecurity, because they ask us to think hard about what kind of future we want. To their credit, Church and his collaborators have engaged bioethicists from the start of their work, as have the pioneers behind CRISPR. But the challenges coming from successful gene editing and writing are too large to be outsourced to professional ethicists. These new technologies offer control over the code of life, but only we as a society can seize control over where these tools will take us.
A robot server, controlled remotely by a disabled worker, delivers drinks to patrons at the DAWN cafe in Tokyo.
A sleek, four-foot tall white robot glides across a cafe storefront in Tokyo’s Nihonbashi district, holding a two-tiered serving tray full of tea sandwiches and pastries. The cafe’s patrons smile and say thanks as they take the tray—but it’s not the robot they’re thanking. Instead, the patrons are talking to the person controlling the robot—a restaurant employee who operates the avatar from the comfort of their home.
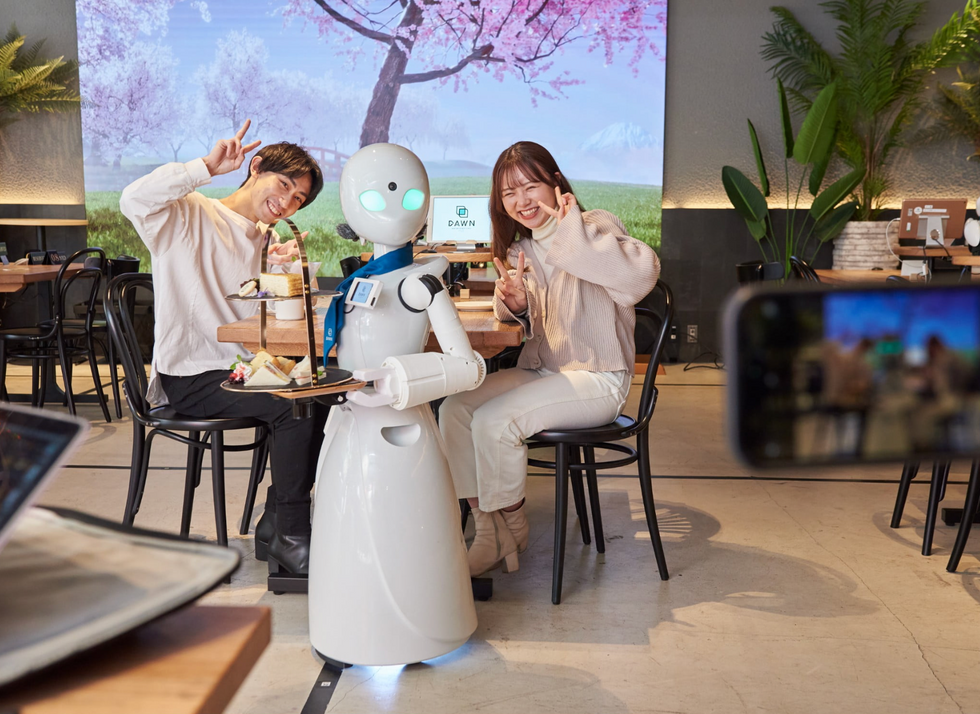
It’s a typical scene at DAWN, short for Diverse Avatar Working Network—a cafe that launched in Tokyo six years ago as an experimental pop-up and quickly became an overnight success. Today, the cafe is a permanent fixture in Nihonbashi, staffing roughly 60 remote workers who control the robots remotely and communicate to customers via a built-in microphone.
More than just a creative idea, however, DAWN is being hailed as a life-changing opportunity. The workers who control the robots remotely (known as “pilots”) all have disabilities that limit their ability to move around freely and travel outside their homes. Worldwide, an estimated 16 percent of the global population lives with a significant disability—and according to the World Health Organization, these disabilities give rise to other problems, such as exclusion from education, unemployment, and poverty.
These are all problems that Kentaro Yoshifuji, founder and CEO of Ory Laboratory, which supplies the robot servers at DAWN, is looking to correct. Yoshifuji, who was bedridden for several years in high school due to an undisclosed health problem, launched the company to help enable people who are house-bound or bedridden to more fully participate in society, as well as end the loneliness, isolation, and feelings of worthlessness that can sometimes go hand-in-hand with being disabled.
“It’s heartbreaking to think that [people with disabilities] feel they are a burden to society, or that they fear their families suffer by caring for them,” said Yoshifuji in an interview in 2020. “We are dedicating ourselves to providing workable, technology-based solutions. That is our purpose.”
Shota, Kuwahara, a DAWN employee with muscular dystrophy, agrees. "There are many difficulties in my daily life, but I believe my life has a purpose and is not being wasted," he says. "Being useful, able to help other people, even feeling needed by others, is so motivational."
A woman receives a mammogram, which can detect the presence of tumors in a patient's breast.
When a patient is diagnosed with early-stage breast cancer, having surgery to remove the tumor is considered the standard of care. But what happens when a patient can’t have surgery?
Whether it’s due to high blood pressure, advanced age, heart issues, or other reasons, some breast cancer patients don’t qualify for a lumpectomy—one of the most common treatment options for early-stage breast cancer. A lumpectomy surgically removes the tumor while keeping the patient’s breast intact, while a mastectomy removes the entire breast and nearby lymph nodes.
Fortunately, a new technique called cryoablation is now available for breast cancer patients who either aren’t candidates for surgery or don’t feel comfortable undergoing a surgical procedure. With cryoablation, doctors use an ultrasound or CT scan to locate any tumors inside the patient’s breast. They then insert small, needle-like probes into the patient's breast which create an “ice ball” that surrounds the tumor and kills the cancer cells.
Cryoablation has been used for decades to treat cancers of the kidneys and liver—but only in the past few years have doctors been able to use the procedure to treat breast cancer patients. And while clinical trials have shown that cryoablation works for tumors smaller than 1.5 centimeters, a recent clinical trial at Memorial Sloan Kettering Cancer Center in New York has shown that it can work for larger tumors, too.
In this study, doctors performed cryoablation on patients whose tumors were, on average, 2.5 centimeters. The cryoablation procedure lasted for about 30 minutes, and patients were able to go home on the same day following treatment. Doctors then followed up with the patients after 16 months. In the follow-up, doctors found the recurrence rate for tumors after using cryoablation was only 10 percent.
For patients who don’t qualify for surgery, radiation and hormonal therapy is typically used to treat tumors. However, said Yolanda Brice, M.D., an interventional radiologist at Memorial Sloan Kettering Cancer Center, “when treated with only radiation and hormonal therapy, the tumors will eventually return.” Cryotherapy, Brice said, could be a more effective way to treat cancer for patients who can’t have surgery.
“The fact that we only saw a 10 percent recurrence rate in our study is incredibly promising,” she said.

