Is Alzheimer's Research On the Wrong Track?

Scientist in laboratory
"The graveyard of hope." That's what experts call the quest for effective Alzheimer's treatments, a two-decade effort that has been marked by one costly and high-profile failure after another. Nearly all of the drugs tested target one of the key hallmarks of Alzheimer's disease: amyloid plaques, the barnacle-like proteins long considered the culprits behind the memory-robbing ravages of the disease. Yet all the anti-amyloid drugs have flopped miserably, prompting some scientists to believe we've fingered the wrong villain.
"We're flogging a dead horse," says Peter Davies, PhD, an Alzheimer's researcher at the Feinstein Institute for Medical Research in New York. "The fact that no one's gotten better suggests that you have the wrong mechanism."
If the naysayers are right, how could a scientific juggernaut of this magnitude—involving hundreds of scientists in academia and industry at a cost of tens of billions of dollars--be so far off the mark? There are no easy answers, but some experts believe this calls into question how research is conducted and blame part of the failure on the insular culture of the scientific aristocracy at leading academic institutions.
"The field began to be dominated by narrow views."
"The field began to be dominated by narrow views," says George Perry, PhD, an Alzheimer's researcher and dean of the College of Sciences at the University of Texas in San Antonio. "The people pushing this were incredibly articulate, powerful and smart. They'd go to scientific meetings and all hang around with each other and they'd self-reinforce."
In fairness, there was solid science driving this. Post-mortem analyses of Alzheimer's patients found their brains were riddled with amyloid plaques. People with a strong family history of Alzheimer's had genetic mutations in the genes that encode for the production of amyloids. And in animal studies, scientists found that if amyloids were inserted into the brains of transgenic mice, they exhibited signs of memory loss. Remove the amyloids and they suddenly got better. This body of research helped launch the Amyloid Cascade Hypothesis of the disease in 1992—which has driven research ever since.
Scientists believed that the increase in the production of these renegade proteins, which form sticky plaques and collect outside of the nerve cells in the brain, triggers a series of events that interfere with the signaling system between synapses. This seems to prevent cells from relaying messages or talking to each other, causing memory loss, confusion and increasing difficulties doing the normal tasks of life. The path forward seemed clear: stop amyloid production and prevent disease progression. "We were going after the obvious abnormality," says Dr. David Knopman, a neurologist and Alzheimer's researcher at the Mayo Clinic in Rochester, Minnesota.
"Why wouldn't you do that?" Why ideed.
In hindsight, though, there was no real smoking gun—no one ever showed precisely how the production of amyloids instigates the destruction of vital brain circuits.
"Amyloids are clearly important," says Perry, "but they have not proven to be necessary and sufficient for the development of this disease."
Ironically, there have been hints all along that amyloids may not be toxic bad boys.
A handful of studies revealed that amyloid proteins are produced in healthy brains to protect synapses. Research on animal models that mimic diseases suggest that certain forms of amyloids can ease damage from strokes, traumatic brain injuries and even heart attacks. In a 2013 study, to cite just one example, a Stanford University team injected synthetic amyloids into paralyzed mice with an inflammatory disorder similar to multiple sclerosis. Instead of worsening their symptoms—which is what the researchers expected to happen--the mice could suddenly walk again. Remove the amyloids, and they became paralyzed once more.
Still other studies suggest amyloids may actually function as molecular guardians dispatched to silence inflammation and mop up errant cells after an injury as part of the body's waste management system. "The presence of amyloids is a protective response to something going wrong, a threat," says Dr. Dale Bredesen, a UCLA neurologist. "But the problem arises when the threats are chronic, multiple, unrelenting and intense. The defenses the brain mounts are also intense and these protective mechanisms cross the line into causing harm, and killing the very synapses and brain cells the amyloid was called up to protect."
So how did research get derailed?
In a way, we're victims of our own success, critics say.
Early medical triumphs in the heady post-World War II era, like the polio vaccine that eradicated the crippling childhood killer, or antibiotics, reinforced the magic bullet idea of curing disease--find a target and then hit it relentlessly. That's why when scientists made the link between amyloids and disease progression, Big Pharma jumped on the bandwagon in hopes of inventing a trillion-dollar drug. This approach is fine when you have an acute illness, like an infectious disease that's caused by one agent, but not for something as complicated as Alzheimer's.
The other piece of the problem is the dwindling federal dollars for basic research. Maverick scientists find it difficult to secure funding, which means that other possible targets or approaches remained relatively unexplored—and drug companies are understandably reluctant to sponsor fishing expeditions with little guarantee of a payoff. "Very influential people were driving this hypothesis," says Davies, and with careers on the line, "there was not enough objectivity or skepticism about that hypothesis."
Still, no one is disputing the importance of anti-amyloid drugs—and ongoing clinical trials, like the DIAN and A4 studies, are intervening earlier in patients who are at a high risk of developing Alzheimer's, but before they're symptomatic. "The only way to know if this is really a dead end is if you take it as far as it can go," says Knopman. "I believe the A4 study is the proper way to test the amyloid hypothesis."
But according to some experts, the latest thinking is that Alzheimer's is triggered by a range of factors, including genetics, poor diet, stress and lack of exercise.
"Alzheimer's is like other chronic age-related diseases and is multi-factorial," says Perry. "Modulating amyloids may have value but other avenues need to be explored."
Thanks to safety cautions from the COVID-19 pandemic, a strain of influenza has been completely eliminated.
If you were one of the millions who masked up, washed your hands thoroughly and socially distanced, pat yourself on the back—you may have helped change the course of human history.
Scientists say that thanks to these safety precautions, which were introduced in early 2020 as a way to stop transmission of the novel COVID-19 virus, a strain of influenza has been completely eliminated. This marks the first time in human history that a virus has been wiped out through non-pharmaceutical interventions, such as vaccines.
The flu shot, explained
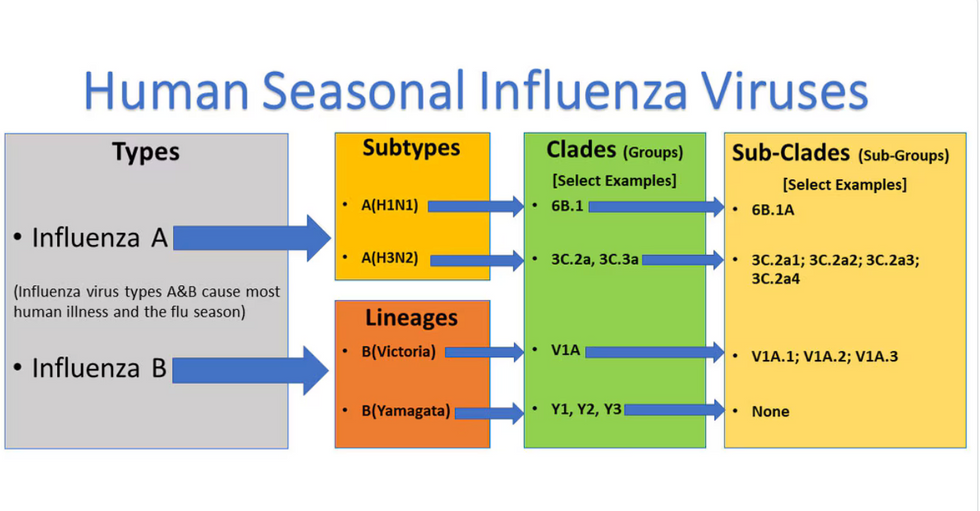
Influenza viruses type A and B are responsible for the majority of human illnesses and the flu season.
Centers for Disease Control
For more than a decade, flu shots have protected against two types of the influenza virus–type A and type B. While there are four different strains of influenza in existence (A, B, C, and D), only strains A, B, and C are capable of infecting humans, and only A and B cause pandemics. In other words, if you catch the flu during flu season, you’re most likely sick with flu type A or B.
Flu vaccines contain inactivated—or dead—influenza virus. These inactivated viruses can’t cause sickness in humans, but when administered as part of a vaccine, they teach a person’s immune system to recognize and kill those viruses when they’re encountered in the wild.
Each spring, a panel of experts gives a recommendation to the US Food and Drug Administration on which strains of each flu type to include in that year’s flu vaccine, depending on what surveillance data says is circulating and what they believe is likely to cause the most illness during the upcoming flu season. For the past decade, Americans have had access to vaccines that provide protection against two strains of influenza A and two lineages of influenza B, known as the Victoria lineage and the Yamagata lineage. But this year, the seasonal flu shot won’t include the Yamagata strain, because the Yamagata strain is no longer circulating among humans.
How Yamagata Disappeared

Flu surveillance data from the Global Initiative on Sharing All Influenza Data (GISAID) shows that the Yamagata lineage of flu type B has not been sequenced since April 2020.
Nature
Experts believe that the Yamagata lineage had already been in decline before the pandemic hit, likely because the strain was naturally less capable of infecting large numbers of people compared to the other strains. When the COVID-19 pandemic hit, the resulting safety precautions such as social distancing, isolating, hand-washing, and masking were enough to drive the virus into extinction completely.
Because the strain hasn’t been circulating since 2020, the FDA elected to remove the Yamagata strain from the seasonal flu vaccine. This will mark the first time since 2012 that the annual flu shot will be trivalent (three-component) rather than quadrivalent (four-component).
Should I still get the flu shot?
The flu shot will protect against fewer strains this year—but that doesn’t mean we should skip it. Influenza places a substantial health burden on the United States every year, responsible for hundreds of thousands of hospitalizations and tens of thousands of deaths. The flu shot has been shown to prevent millions of illnesses each year (more than six million during the 2022-2023 season). And while it’s still possible to catch the flu after getting the flu shot, studies show that people are far less likely to be hospitalized or die when they’re vaccinated.
Another unexpected benefit of dropping the Yamagata strain from the seasonal vaccine? This will possibly make production of the flu vaccine faster, and enable manufacturers to make more vaccines, helping countries who have a flu vaccine shortage and potentially saving millions more lives.
After his grandmother’s dementia diagnosis, one man invented a snack to keep her healthy and hydrated.
Founder Lewis Hornby and his grandmother Pat, sampling Jelly Drops—an edible gummy containing water and life-saving electrolytes.
On a visit to his grandmother’s nursing home in 2016, college student Lewis Hornby made a shocking discovery: Dehydration is a common (and dangerous) problem among seniors—especially those that are diagnosed with dementia.
Hornby’s grandmother, Pat, had always had difficulty keeping up her water intake as she got older, a common issue with seniors. As we age, our body composition changes, and we naturally hold less water than younger adults or children, so it’s easier to become dehydrated quickly if those fluids aren’t replenished. What’s more, our thirst signals diminish naturally as we age as well—meaning our body is not as good as it once was in letting us know that we need to rehydrate. This often creates a perfect storm that commonly leads to dehydration. In Pat’s case, her dehydration was so severe she nearly died.
When Lewis Hornby visited his grandmother at her nursing home afterward, he learned that dehydration especially affects people with dementia, as they often don’t feel thirst cues at all, or may not recognize how to use cups correctly. But while dementia patients often don’t remember to drink water, it seemed to Hornby that they had less problem remembering to eat, particularly candy.

Hornby wanted to create a solution for elderly people who struggled keeping their fluid intake up. He spent the next eighteen months researching and designing a solution and securing funding for his project. In 2019, Hornby won a sizable grant from the Alzheimer’s Society, a UK-based care and research charity for people with dementia and their caregivers. Together, through the charity’s Accelerator Program, they created a bite-sized, sugar-free, edible jelly drop that looked and tasted like candy. The candy, called Jelly Drops, contained 95% water and electrolytes—important minerals that are often lost during dehydration. The final product launched in 2020—and was an immediate success. The drops were able to provide extra hydration to the elderly, as well as help keep dementia patients safe, since dehydration commonly leads to confusion, hospitalization, and sometimes even death.
Not only did Jelly Drops quickly become a favorite snack among dementia patients in the UK, but they were able to provide an additional boost of hydration to hospital workers during the pandemic. In NHS coronavirus hospital wards, patients infected with the virus were regularly given Jelly Drops to keep their fluid levels normal—and staff members snacked on them as well, since long shifts and personal protective equipment (PPE) they were required to wear often left them feeling parched.
In April 2022, Jelly Drops launched in the United States. The company continues to donate 1% of its profits to help fund Alzheimer’s research.

