Is China Winning the Innovation Race?

Flags of America and China illuminated by light bulbs with a question mark between them.
Over the past two millennia, Chinese ingenuity has spawned some of humanity's most consequential inventions. Without gunpowder, guns, bombs, and rockets; without paper, printing, and money printed on paper; and without the compass, which enabled ships to navigate the open ocean, modern civilization might never have been born.
Today, a specter is haunting the developed world: Chinese innovation dominance. And the results have been so spectacular that the United States feels its preeminence threatened.
Yet China lapsed into cultural and technological stagnation during the Qing dynasty, just as the Scientific Revolution was transforming Europe. Western colonial incursions and a series of failed rebellions further sapped the Celestial Empire's capacity for innovation. By the mid-20th century, when the Communist triumph led to a devastating famine and years of bloody political turmoil, practically the only intellectual property China could offer for export was Mao's Little Red Book.
After Deng Xiaoping took power in 1978, launching a transition from a rigidly planned economy to a semi-capitalist one, China's factories began pumping out goods for foreign consumption. Still, originality remained a low priority. The phrase "Made in China" came to be synonymous with "cheap knockoff."
Today, however, a specter is haunting the developed world: Chinese innovation dominance. It first wafted into view in 2006, when the government announced an "indigenous innovation" campaign, dedicated to establishing China as a technology powerhouse by 2020—and a global leader by 2050—as part of its Medium- and Long-Term National Plan for Science and Technology Development. Since then, an array of initiatives have sought to unleash what pundits often call the Chinese "tech dragon," whether in individual industries, such as semiconductors or artificial intelligence, or across the board (as with the Made in China 2025 project, inaugurated in 2015). These efforts draw on a well-stocked bureaucratic arsenal: state-directed financing; strategic mergers and acquisitions; competition policies designed to boost domestic companies and hobble foreign rivals; buy-Chinese procurement policies; cash incentives for companies to file patents; subsidies for academic researchers in favored fields.
The results have been spectacular—so much so that the United States feels its preeminence threatened. Voices across the political spectrum are calling for emergency measures, including a clampdown on technology transfers, capital investment, and Chinese students' ability to study abroad. But are the fears driving such proposals justified?
"We've flipped from thinking China is incapable of anything but imitation to thinking China is about to eat our lunch," says Kaiser Kuo, host of the Sinica podcast at supchina.com, who recently returned to the U.S after 20 years in Beijing—the last six as director of international communications for the tech giant Baidu. Like some other veteran China-watchers, Kuo believes neither extreme reflects reality. "We're in as much danger now of overestimating China's innovative capacity," he warns, "as we were a few years ago of underestimating it."
A Lab and Tech-Business Bonanza
By many measures, China's innovation renaissance is mind-boggling. Spending on research and development as a percentage of gross domestic product nearly quadrupled between 1996 and 2016, from .56 percent to 2.1 percent; during the same period, spending in the United States rose by just .3 percentage points, from 2.44 to 2.79 percent of GDP. China is now second only to the U.S. in total R&D spending, accounting for 21 percent of the global total of $2 trillion, according to a report released in January by the National Science Foundation. In 2016, the number of scientific publications from China exceeded those from the U.S. for the first time, by 426,000 to 409,000. Chinese researchers are blazing new trails on the frontiers of cloning, stem cell medicine, gene editing, and quantum computing. Chinese patent applications have soared from 170,000 to nearly 3 million since 2000; the country now files almost as many international patents as the U.S. and Japan, and more than Germany and South Korea. Between 2008 and 2017, two Chinese tech firms—Huawei and ZTE—traded places as the world's top patent filer in six out of nine years.
"China is still in its Star Trek phase, while we're in our Black Mirror phase." Yet there are formidable barriers to China beating America in the innovation race—or even catching up anytime soon.
Accompanying this lab-based ferment is a tech-business bonanza. China's three biggest internet companies, Baidu, Alibaba Group and Tencent Holdings (known collectively as BAT), have become global titans of search, e-commerce, mobile payments, gaming, and social media. Da-Jiang Innovations in Science and Technology (DJI) controls more than 70 percent of the world's commercial drone market. Of the planet's 262 "unicorns" (startups worth more than a billion dollars), about one-third are Chinese. The country attracted $77 billion in venture capital investment between 2014 and 2016, according to Fortune, and is now among the top three markets for VC in emerging technologies including AI, virtual reality, autonomous vehicles, and 3D printing.
These developments have fueled a buoyant techno-optimism in China that contrasts sharply with the darker view increasingly prevalent in the West—in part, perhaps, because China's historic limits on civil liberties have inured the populace to the intrusive implications of, say, facial recognition technology or social-credit software, which are already being used to tighten government control. "China is still in its Star Trek phase, while we're in our Black Mirror phase," Kuo observes. By contrast with Americans' ambivalent attitudes toward Facebook founder Mark Zuckerberg or Amazon's Jeff Bezos, he adds, most Chinese regard tech entrepreneurs like Baidu's Robin Li and Alibaba's Jack Ma as "flat-out heroes."
Yet there are formidable barriers to China beating America in the innovation race—or even catching up anytime soon. Many are catalogued in The Fat Tech Dragon, a 2017 monograph by Scott Kennedy, deputy director of the Freeman Chair in China Studies and director of the Project on Chinese Business and Political Economy at the Center for Strategic and International Studies. Among the obstacles, Kennedy writes, are "an education system that encourages deference to authority and does not prepare students to be creative and take risks, a financial system that disproportionately funnels funds to undeserving state-owned enterprises… and a market structure where profits can be made through a low-margin, high-volume strategy or through political connections."
China's R&D money, Kennedy points out, is mostly showered on the "D": of the $209 billion spent in 2015, only 5 percent went toward basic research, 10.8 percent toward applied research, and a massive 84.2 percent toward development. While fully half of venture capital in the States goes to early-stage startups, the figure for China is under 20 percent; true "angel" investors are scarce. Likewise, only 21 percent of Chinese patents are for original inventions, as opposed to tweaks of existing technologies. Most problematic, the domestic value of patents in China is strikingly low. In 2015, the country's patent licensing generated revenues of just $1.75 billion, compared to $115 billion for IP licensing in the U.S. in 2012 (the most recent year for which data is available). In short, Kennedy concludes, "China may now be a 'large' IP country, but it is still a 'weak' one."
"[The Chinese] are trying very hard to keep the economy from crashing, but it'll happen eventually. Then there will be a major, major contraction."
Anne Stevenson-Yang, co-founder and research director of J Capital Research, and a leading China analyst, sees another potential stumbling block: the government's obsession with neck-snapping GDP growth. "What China does is to determine, 'Our GDP growth will be X,' and then it generates enough investment to create X," Stevenson-Yang explains. To meet those quotas, officials pour money into gigantic construction projects, creating the empty "ghost cities" that litter the countryside, or subsidize industrial production far beyond realistic demand. "It's the ultimate Ponzi-scheme economy," she says, citing as examples the Chinese cellphone and solar industries, which ballooned on state funding, flooded global markets with dirt-cheap products, thrived just long enough to kill off most of their overseas competitors, and then largely collapsed. Such ventures, Stevenson-Yang notes, have driven China's debt load perilously high. "They're trying very hard to keep the economy from crashing, but it'll happen eventually," she predicts. "Then there will be a major, major contraction."
"An Intensifying Race Toward Techno-Nationalism"
The greatest vulnerability of the Chinese innovation boom may be that it still depends heavily on imported IP. "Over the last few years, China has placed its bets on a combination of global knowledge sourcing and indigenous technology development," says Dieter Ernst, a senior fellow at the Centre for International Governance Innovation in Waterloo, Canada, and the East-West Center in Honolulu, who has served as an Asia advisor for the U.N. and the World Bank. Aside from international journals (and, occasionally, industrial espionage), Chinese labs and corporations obtain non-indigenous knowledge in a number of ways: by paying licensing fees; recruiting Chinese scientists and engineers who've studied or worked abroad; hiring professionals from other countries; or acquiring foreign companies. And though enforcement of IP laws has improved markedly in recent years, foreign businesses are often pressured to provide technology transfers in exchange for access to markets.
Many of China's top tech entrepreneurs—including Ma, Li, and Alibaba's Joseph Tsai—are alumni of U.S. universities, and, as Kuo puts it, "big fans of all things American." Unfortunately, however, Americans are ever less likely to be fans of China, thanks largely to that country's sometimes predatory trade practices—and also to what Ernst calls "an intensifying race toward techno-nationalism." With varying degrees of bellicosity and consistency, leaders of both U.S. parties embrace elements of the trend, as do politicians (and voters) across much of Europe. "There's a growing consensus that China is poised to overtake us," says Ernst, "and that we need to design policies to obstruct its rise."
One of the foremost liberal analysts supporting this view is Lee Branstetter, a professor of economics and public policy at Carnegie Mellon University and former senior economist on President Barack Obama's Council of Economic Advisors. "Over the decades, in a systematic and premeditated fashion, the Chinese government and its state-owned enterprises have worked to extract valuable technology from foreign multinationals, with an explicit goal of eventually displacing those leading multinationals with successful Chinese firms in global markets," Branstetter wrote in a 2017 report to the United States Trade Representative. To combat such "forced transfers," he suggested, laws could be passed empowering foreign governments to investigate coercive requests and block any deemed inappropriate—not just those involving military-related or crucial infrastructure technology, which current statutes cover. Branstetter also called for "sharply" curtailing Chinese students' access to Western graduate programs, as a way to "get policymakers' attention in Beijing" and induce them to play fair.
Similar sentiments are taking hold in Congress, where the Foreign Investment Risk Review Modernization Act—aimed at strengthening the process by which the Committee on Foreign Investment in the United States reviews Chinese acquisition of American technologies—is expected to pass with bipartisan support, though its harsher provisions were softened due to objections from Silicon Valley. The Trump Administration announced in May that it would soon take executive action to curb Chinese investments in U.S. tech firms and otherwise limit access to intellectual property. The State Department, meanwhile, imposed a one-year limit on visas for Chinese grad students in high-tech fields.
Ernst argues that such measures are motivated largely by exaggerated notions of China's ability to reach its ambitious goals, and by the political advantages that fearmongering confers. "If you look at AI, chip design and fabrication, robotics, pharmaceuticals, the gap with the U.S. is huge," he says. "Reducing it will take at least 10 or 15 years."
Cracking down on U.S. tech transfers to Chinese companies, Ernst cautions, will deprive U.S. firms of vital investment capital and spur China to retaliate, cutting off access to the nation's gargantuan markets; it will also push China to forge IP deals with more compliant nations, or revert to outright piracy. And restricting student visas, besides harming U.S. universities that depend on Chinese scholars' billions in tuition, will have a "chilling effect on America's ability to attract to researchers and engineers from all countries."
"It's not a zero-sum game. I don't think China is going to eat our lunch. We can sit down and enjoy lunch together."
America's own science and technology community, Ernst adds, considers it crucial to swap ideas with China's fast-growing pool of talent. The 2017 annual meeting of the Palo Alto-based Association for Advancement of Artificial Intelligence, he notes, featured a nearly equal number of papers by researchers in China and the U.S. Organizers postponed the meeting after discovering that the original date coincided with the Chinese New Year.
China's rising influence on the tech world carries upsides as well as downsides, Scott Kennedy observes. The country's successes in e-commerce, he says, "haven't damaged the global internet sector, but have actually been a spur to additional innovation and progress. By contrast, China's success in solar and wind has decimated the global sectors," due to state-mandated overcapacity. "When Chinese firms win through open competition, the outcome is constructive; when they win through industrial policy and protectionism, the outcome is destructive."
The solution, Kennedy and like-minded experts argue, is to discourage protectionism rather than engage in it, adjusting tech-transfer policy just enough to cope with evolving national-security concerns. Instead of trying to squelch China's innovation explosion, they say, the U.S. should seek ways to spread its potential benefits (as happened in previous eras with Japan and South Korea), and increase America's indigenous investments in tech-related research, education, and job training.
"It's not a zero-sum game," says Kaiser Kuo. "I don't think China is going to eat our lunch. We can sit down and enjoy lunch together."
Meet Dr. Renee Wegrzyn, the first Director of President Biden's new health agency, ARPA-H
Today's podcast guest, Dr. Renee Wegrzyn, directs ARPA-H, a new agency formed last year to spearhead health innovations. Time will tell if ARPA-H will produce advances on the level of its fellow agency, DARPA.
In today’s podcast episode, I talk with Renee Wegrzyn, appointed by President Biden as the first director of a health agency created last year, the Advanced Research Projects Agency for Health, or ARPA-H. It’s inspired by DARPA, the agency that develops innovations for the Defense department and has been credited with hatching world-changing technologies such as ARPANET, which became the internet.
Time will tell if ARPA-H will lead to similar achievements in the realm of health. That’s what President Biden and Congress expect in return for funding ARPA-H at 2.5 billion dollars over three years.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
How will the agency figure out which projects to take on, especially with so many patient advocates for different diseases demanding moonshot funding for rapid progress?
I talked with Dr. Wegrzyn about the opportunities and challenges, what lessons ARPA-H is borrowing from Operation Warp Speed, how she decided on the first ARPA-H project that was announced recently, why a separate agency was needed instead of reforming HHS and the National Institutes of Health to be better at innovation, and how ARPA-H will make progress on disease prevention in addition to treatments for cancer, Alzheimer’s and diabetes, among many other health priorities.
Dr. Wegrzyn’s resume leaves no doubt of her suitability for this role. She was a program manager at DARPA where she focused on applying gene editing and synthetic biology to the goal of improving biosecurity. For her work there, she received the Superior Public Service Medal and, in case that wasn’t enough ARPA experience, she also worked at another ARPA that leads advanced projects in intelligence, called I-ARPA. Before that, she ran technical teams in the private sector working on gene therapies and disease diagnostics, among other areas. She has been a vice president of business development at Gingko Bioworks and headed innovation at Concentric by Gingko. Her training and education includes a PhD and undergraduate degree in applied biology from the Georgia Institute of Technology and she did her postdoc as an Alexander von Humboldt Fellow in Heidelberg, Germany.
Dr. Wegrzyn told me that she’s “in the hot seat.” The pressure is on for ARPA-H especially after the need and potential for health innovation was spot lit by the pandemic and the unprecedented speed of vaccine development. We'll soon find out if ARPA-H can produce gamechangers in health that are equivalent to DARPA’s creation of the internet.
Show links:
ARPA-H - https://arpa-h.gov/
Dr. Wegrzyn profile - https://arpa-h.gov/people/renee-wegrzyn/
Dr. Wegrzyn Twitter - https://twitter.com/rwegrzyn?lang=en
President Biden Announces Dr. Wegrzyn's appointment - https://www.whitehouse.gov/briefing-room/statement...
Leaps.org coverage of ARPA-H - https://leaps.org/arpa/
ARPA-H program for joints to heal themselves - https://arpa-h.gov/news/nitro/ -
ARPA-H virtual talent search - https://arpa-h.gov/news/aco-talent-search/

Dr. Renee Wegrzyn was appointed director of ARPA-H last October.
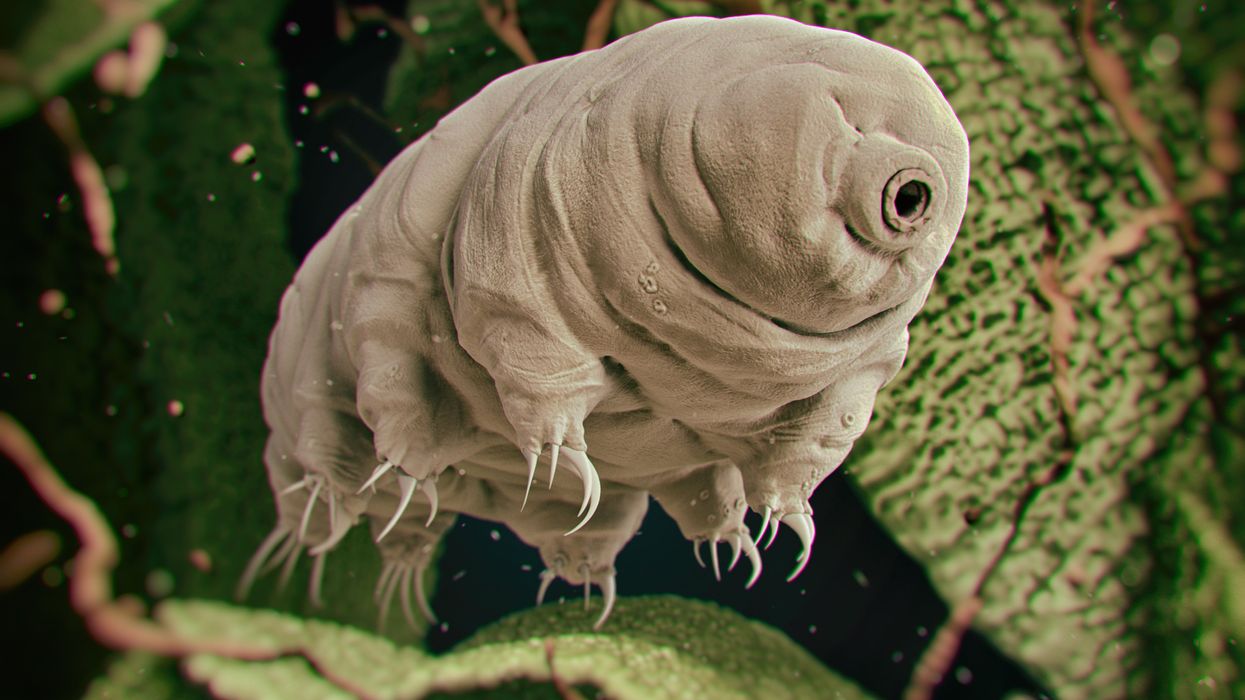
Tiny, tough “water bears” may help bring new vaccines and medicines to sub-Saharan Africa
Tardigrades can completely dehydrate and later rehydrate themselves, a survival trick that scientists are harnessing to preserve medicines in hot temperatures.
Microscopic tardigrades, widely considered to be some of the toughest animals on earth, can survive for decades without oxygen or water and are thought to have lived through a crash-landing on the moon. Also known as water bears, they survive by fully dehydrating and later rehydrating themselves – a feat only a few animals can accomplish. Now scientists are harnessing tardigrades’ talents to make medicines that can be dried and stored at ambient temperatures and later rehydrated for use—instead of being kept refrigerated or frozen.
Many biologics—pharmaceutical products made by using living cells or synthesized from biological sources—require refrigeration, which isn’t always available in many remote locales or places with unreliable electricity. These products include mRNA and other vaccines, monoclonal antibodies and immuno-therapies for cancer, rheumatoid arthritis and other conditions. Cooling is also needed for medicines for blood clotting disorders like hemophilia and for trauma patients.
Formulating biologics to withstand drying and hot temperatures has been the holy grail for pharmaceutical researchers for decades. It’s a hard feat to manage. “Biologic pharmaceuticals are highly efficacious, but many are inherently unstable,” says Thomas Boothby, assistant professor of molecular biology at University of Wyoming. Therefore, during storage and shipping, they must be refrigerated at 2 to 8 degrees Celsius (35 to 46 degrees Fahrenheit). Some must be frozen, typically at -20 degrees Celsius, but sometimes as low -90 degrees Celsius as was the case with the Pfizer Covid vaccine.
For Covid, fewer than 73 percent of the global population received even one dose. The need for refrigerated or frozen handling was partially to blame.
The costly cold chain
The logistics network that ensures those temperature requirements are met from production to administration is called the cold chain. This cold chain network is often unreliable or entirely lacking in remote, rural areas in developing nations that have malfunctioning electrical grids. “Almost all routine vaccines require a cold chain,” says Christopher Fox, senior vice president of formulations at the Access to Advanced Health Institute. But when the power goes out, so does refrigeration, putting refrigerated or frozen medical products at risk. Consequently, the mRNA vaccines developed for Covid-19 and other conditions, as well as more traditional vaccines for cholera, tetanus and other diseases, often can’t be delivered to the most remote parts of the world.
To understand the scope of the challenge, consider this: In the U.S., more than 984 million doses of Covid-19 vaccine have been distributed so far. Each one needed refrigeration that, even in the U.S., proved challenging. Now extrapolate to all vaccines and the entire world. For Covid, fewer than 73 percent of the global population received even one dose. The need for refrigerated or frozen handling was partially to blame.
Globally, the cold chain packaging market is valued at over $15 billion and is expected to exceed $60 billion by 2033.
Adobe Stock
Freeze-drying, also called lyophilization, which is common for many vaccines, isn’t always an option. Many freeze-dried vaccines still need refrigeration, and even medicines approved for storage at ambient temperatures break down in the heat of sub-Saharan Africa. “Even in a freeze-dried state, biologics often will undergo partial rehydration and dehydration, which can be extremely damaging,” Boothby explains.
The cold chain is also very expensive to maintain. The global pharmaceutical cold chain packaging market is valued at more than $15 billion, and is expected to exceed $60 billion by 2033, according to a report by Future Market Insights. This cost is only expected to grow. According to the consulting company Accenture, the number of medicines that require the cold chain are expected to grow by 48 percent, compared to only 21 percent for non-cold-chain therapies.
Tardigrades to the rescue
Tardigrades are only about a millimeter long – with four legs and claws, and they lumber around like bears, thus their nickname – but could provide a big solution. “Tardigrades are unique in the animal kingdom, in that they’re able to survive a vast array of environmental insults,” says Boothby, the Wyoming professor. “They can be dried out, frozen, heated past the boiling point of water and irradiated at levels that are thousands of times more than you or I could survive.” So, his team is gradually unlocking tardigrades’ survival secrets and applying them to biologic pharmaceuticals to make them withstand both extreme heat and desiccation without losing efficacy.
Boothby’s team is focusing on blood clotting factor VIII, which, as the name implies, causes blood to clot. Currently, Boothby is concentrating on the so-called cytoplasmic abundant heat soluble (CAHS) protein family, which is found only in tardigrades, protecting them when they dry out. “We showed we can desiccate a biologic (blood clotting factor VIII, a key clotting component) in the presence of tardigrade proteins,” he says—without losing any of its effectiveness.
The researchers mixed the tardigrade protein with the blood clotting factor and then dried and rehydrated that substance six times without damaging the latter. This suggests that biologics protected with tardigrade proteins can withstand real-world fluctuations in humidity.
Furthermore, Boothby’s team found that when the blood clotting factor was dried and stabilized with tardigrade proteins, it retained its efficacy at temperatures as high as 95 degrees Celsius. That’s over 200 degrees Fahrenheit, much hotter than the 58 degrees Celsius that the World Meteorological Organization lists as the hottest recorded air temperature on earth. In contrast, without the protein, the blood clotting factor degraded significantly. The team published their findings in the journal Nature in March.
Although tardigrades rarely live more than 2.5 years, they have survived in a desiccated state for up to two decades, according to Animal Diversity Web. This suggests that tardigrades’ CAHS protein can protect biologic pharmaceuticals nearly indefinitely without refrigeration or freezing, which makes it significantly easier to deliver them in locations where refrigeration is unreliable or doesn’t exist.
The tricks of the tardigrades
Besides the CAHS proteins, tardigrades rely on a type of sugar called trehalose and some other protectants. So, rather than drying up, their cells solidify into rigid, glass-like structures. As that happens, viscosity between cells increases, thereby slowing their biological functions so much that they all but stop.
Now Boothby is combining CAHS D, one of the proteins in the CAHS family, with trehalose. He found that CAHS D and trehalose each protected proteins through repeated drying and rehydrating cycles. They also work synergistically, which means that together they might stabilize biologics under a variety of dry storage conditions.
“We’re finding the protective effect is not just additive but actually is synergistic,” he says. “We’re keen to see if something like that also holds true with different protein combinations.” If so, combinations could possibly protect against a variety of conditions.
Commercialization outlook
Before any stabilization technology for biologics can be commercialized, it first must be approved by the appropriate regulators. In the U.S., that’s the U.S. Food and Drug Administration. Developing a new formulation would require clinical testing and vast numbers of participants. So existing vaccines and biologics likely won’t be re-formulated for dry storage. “Many were developed decades ago,” says Fox. “They‘re not going to be reformulated into thermo-stable vaccines overnight,” if ever, he predicts.
Extending stability outside the cold chain, even for a few days, can have profound health, environmental and economic benefits.
Instead, this technology is most likely to be used for the new products and formulations that are just being created. New and improved vaccines will be the first to benefit. Good candidates include the plethora of mRNA vaccines, as well as biologic pharmaceuticals for neglected diseases that affect parts of the world where reliable cold chain is difficult to maintain, Boothby says. Some examples include new, more effective vaccines for malaria and for pathogenic Escherichia coli, which causes diarrhea.
Tallying up the benefits
Extending stability outside the cold chain, even for a few days, can have profound health, environmental and economic benefits. For instance, MenAfriVac, a meningitis vaccine (without tardigrade proteins) developed for sub-Saharan Africa, can be stored at up to 40 degrees Celsius for four days before administration. “If you have a few days where you don’t need to maintain the cold chain, it’s easier to transport vaccines to remote areas,” Fox says, where refrigeration does not exist or is not reliable.
Better health is an obvious benefit. MenAfriVac reduced suspected meningitis cases by 57 percent in the overall population and more than 99 percent among vaccinated individuals.
Lower healthcare costs are another benefit. One study done in Togo found that the cold chain-related costs increased the per dose vaccine price up to 11-fold. The ability to ship the vaccines using the usual cold chain, but transporting them at ambient temperatures for the final few days cut the cost in half.
There are environmental benefits, too, such as reducing fuel consumption and greenhouse gas emissions. Cold chain transports consume 20 percent more fuel than non-cold chain shipping, due to refrigeration equipment, according to the International Trade Administration.
A study by researchers at Johns Hopkins University compared the greenhouse gas emissions of the new, oral Vaxart COVID-19 vaccine (which doesn’t require refrigeration) with four intramuscular vaccines (which require refrigeration or freezing). While the Vaxart vaccine is still in clinical trials, the study found that “up to 82.25 million kilograms of CO2 could be averted by using oral vaccines in the U.S. alone.” That is akin to taking 17,700 vehicles out of service for one year.
Although tardigrades’ protective proteins won’t be a component of biologic pharmaceutics for several years, scientists are proving that this approach is viable. They are hopeful that a day will come when vaccines and biologics can be delivered anywhere in the world without needing refrigerators or freezers en route.