This Resistance Fighter Invented Dialysis in Nazi-Occupied Holland
When Willem Johan Kolff invented dialysis, the "father" of artificial organs was just getting started.
One of the Netherlands’ most famous pieces of pop culture is “Soldier of Orange.” It’s the title of the country’s most celebrated war memoir, movie and epic stage musical, all of which detail the exploits of the nation’s resistance fighters during World War II.
Willem Johan Kolff was a member of the Dutch resistance, but he doesn’t rate a mention in the “Solider of Orange” canon. Yet his wartime toils in a rural backwater not only changed medicine, but the world.
Kolff had been a physician less than two years before Germany invaded the Netherlands in May 1940. He had been engaged in post-graduate studies at the University of Gronigen but withdrew because he refused to accommodate the demands of the Nazi occupiers. Kolff’s Jewish supervisor made an even starker choice: He committed suicide.
After his departure from the university, Kolff took a job managing a small hospital in Kampen. Located 50 miles from the heavily populated coastal region, the facility was far enough away from the prying eyes of Germans that not only could Kolff care for patients, he could hide fellow resistance fighters and even Jewish refugees in relative safety. Kolff coached many of them to feign convincing terminal illnesses so the Nazis would allow them to remain in the hospital.
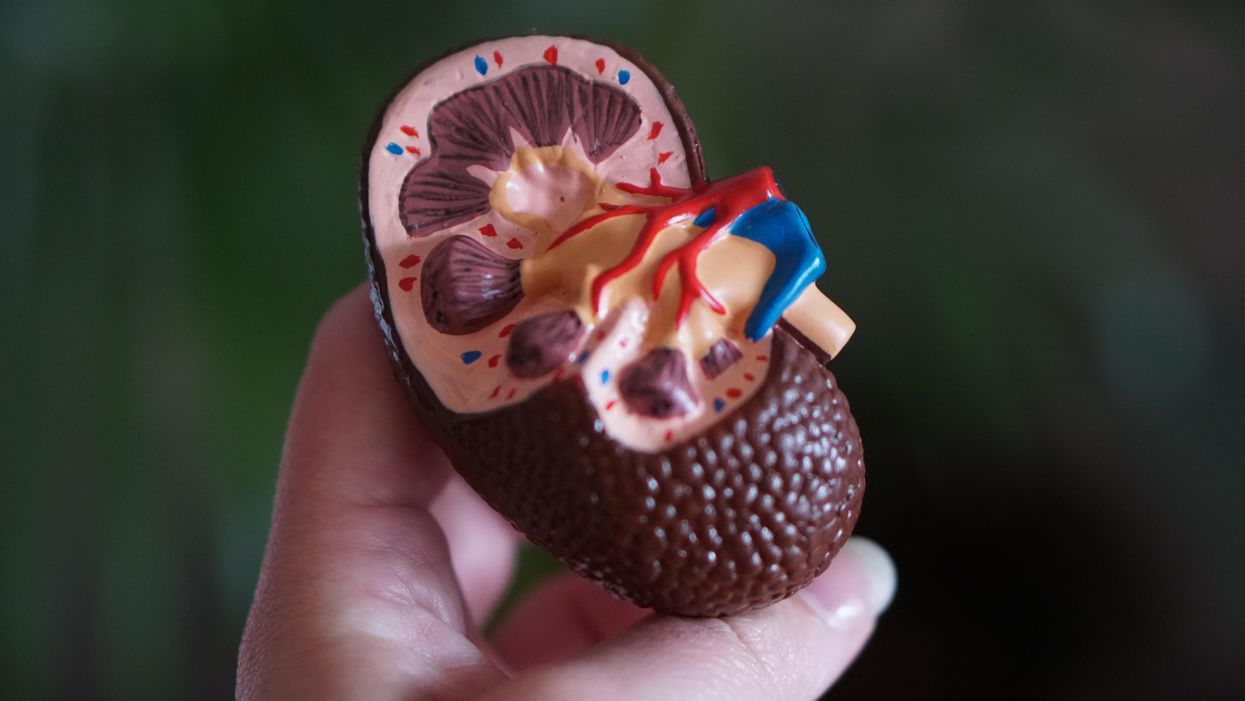
Despite the demands of practicing medicine and resistance work, Kolff still found time to conduct research. He had been haunted and inspired when, not long before the Nazi invasion, one of his patients died in agony from kidney disease. Kolff wanted to find a way to save future patients.
He broke his problem down to a simple task: If he could remove 20 grams of urea from a patient’s blood in 24 hours, they would survive. He began experimenting with ways to filter blood and return it to a patient’s body. Since the war had ground all non-military manufacturing to a halt, he was mostly forced to make do with material he could find at the hospital and around Kampen. Kolff eventually built a device from a washing machine parts, juice cans, sausage casings, a valve from an old Ford automobile radiator, and even scrap from a downed German aircraft.
The world’s first dialysis machine was hardly imposing; it resembled a rotating drum for a bingo game or raffle. Yet it carried on the highly sophisticated task of moving a patient’s blood through a semi-permeable membrane (about a 50-foot length of sausage casings) into a saline solution that drew out urea while leaving the blood cells untouched.
In emigrating to the U.S. to practice medicine, Kolff's intent was twofold: Advocate for a wider adoption of dialysis, and work on new projects. He wildly succeeded at both.
Kolff began using the machine to treat patients in 1943, most of whom had lapsed into comas due to their kidney failure. But like most groundbreaking medical devices, it was not an immediate success. By the end of the war, Kolff had dialyzed more than a dozen patients, but all had died. He briefly suspended use of the device after the Allied invasion of Europe, but he continued to refine its operation and the administration of blood thinners to patients.
In September 1945, Kolff dialyzed another comatose patient, 67-year-old Sofia Maria Schafstadt. She regained consciousness after 11 hours, and would live well into the 1950s with Kolff’s assistance. Yet this triumph contained a dark irony: At the time of her treatment, Schafstadt had been imprisoned for collaborating with the Germans.
With a tattered Europe struggling to overcome the destruction of the war, Kolff and his family emigrated to the U.S. in 1950, where he began working for the Cleveland Clinic while undergoing the naturalization process so he could practice medicine in the U.S. His intent was twofold: Advocate for a wider adoption of dialysis, and work on new projects. He wildly succeeded at both.
By the mid-1950s, dialysis machines had become reliable and life-saving medical devices, and Kolff had become a U.S. citizen. About that time he invented a membrane oxygenator that could be used in heart bypass surgeries. This was a critical component of the heart-lung machine, which would make heart transplants possible and bypass surgeries routine. He also invented among the very first practical artificial hearts, which in 1957 kept a dog alive for 90 minutes.
Kolff moved to the University of Utah in 1967 to become director of its Institute for Biomedical Engineering. It was a promising time for such a move, as the first successful transplant of a donor heart to a human occurred that year. But he was interested in going a step further and creating an artificial heart for human use.
It took more than a decade of tinkering and research, but in 1982, a team of physicians and engineers led by Kolff succeeded in implanting the first artificial heart in dentist Barney Clark, whose failing health disqualified him from a heart transplant. Although Clark died in March 1983 after 112 days tethered to the device, that it kept him alive generated international headlines. While graduate student Robert Jarvik received the named credit for the heart, he was directly supervised by Kolff, whose various endeavors into artificial organ research at the University of Utah were segmented into numerous teams.
Forty years later, several artificial hearts have been approved for use by the Food and Drug Administration, although all are a “bridge” that allow patients to wait for a transplant.
Kolff continued researching and tinkering with biomedical devices – including artificial eyes and ears – until he retired in 1997 at the age of 86. When he died in 2009, the medical community acknowledged that he was not only a pioneer in biotechnology, but the “father” of artificial organs.
Have You Heard of the Best Sport for Brain Health?
In this week's Friday Five, research points to this brain healthiest of sports. Plus, the natural way to reprogram cells to a younger state, the network that could underlie many different mental illnesses, and a new test could diagnose autism in newborns. Plus, scientists 3D print an ear and attach it to woman
The Friday Five covers five stories in research that you may have missed this week. There are plenty of controversies and troubling ethical issues in science – and we get into many of them in our online magazine – but this news roundup focuses on scientific creativity and progress to give you a therapeutic dose of inspiration headed into the weekend.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
Here are the promising studies covered in this week's Friday Five:
- Reprogram cells to a younger state
- Pick up this sport for brain health
- Do all mental illnesses have the same underlying cause?
- New test could diagnose autism in newborns
- Scientists 3D print an ear and attach it to woman
Can blockchain help solve the Henrietta Lacks problem?
Marielle Gross, a professor at the University of Pittsburgh, shows patients a new app that tracks how their samples are used during biomedical research.
Science has come a long way since Henrietta Lacks, a Black woman from Baltimore, succumbed to cervical cancer at age 31 in 1951 -- only eight months after her diagnosis. Since then, research involving her cancer cells has advanced scientific understanding of the human papilloma virus, polio vaccines, medications for HIV/AIDS and in vitro fertilization.
Today, the World Health Organization reports that those cells are essential in mounting a COVID-19 response. But they were commercialized without the awareness or permission of Lacks or her family, who have filed a lawsuit against a biotech company for profiting from these “HeLa” cells.
While obtaining an individual's informed consent has become standard procedure before the use of tissues in medical research, many patients still don’t know what happens to their samples. Now, a new phone-based app is aiming to change that.
Tissue donors can track what scientists do with their samples while safeguarding privacy, through a pilot program initiated in October by researchers at the Johns Hopkins Berman Institute of Bioethics and the University of Pittsburgh’s Institute for Precision Medicine. The program uses blockchain technology to offer patients this opportunity through the University of Pittsburgh's Breast Disease Research Repository, while assuring that their identities remain anonymous to investigators.
A blockchain is a digital, tamper-proof ledger of transactions duplicated and distributed across a computer system network. Whenever a transaction occurs with a patient’s sample, multiple stakeholders can track it while the owner’s identity remains encrypted. Special certificates called “nonfungible tokens,” or NFTs, represent patients’ unique samples on a trusted and widely used blockchain that reinforces transparency.
Blockchain could be used to notify people if cancer researchers discover that they have certain risk factors.
“Healthcare is very data rich, but control of that data often does not lie with the patient,” said Julius Bogdan, vice president of analytics for North America at the Healthcare Information and Management Systems Society (HIMSS), a Chicago-based global technology nonprofit. “NFTs allow for the encapsulation of a patient’s data in a digital asset controlled by the patient.” He added that this technology enables a more secure and informed method of participating in clinical and research trials.
Without this technology, de-identification of patients’ samples during biomedical research had the unintended consequence of preventing them from discovering what researchers find -- even if that data could benefit their health. A solution was urgently needed, said Marielle Gross, assistant professor of obstetrics, gynecology and reproductive science and bioethics at the University of Pittsburgh School of Medicine.
“A researcher can learn something from your bio samples or medical records that could be life-saving information for you, and they have no way to let you or your doctor know,” said Gross, who is also an affiliate assistant professor at the Berman Institute. “There’s no good reason for that to stay the way that it is.”
For instance, blockchain could be used to notify people if cancer researchers discover that they have certain risk factors. Gross estimated that less than half of breast cancer patients are tested for mutations in BRCA1 and BRCA2 — tumor suppressor genes that are important in combating cancer. With normal function, these genes help prevent breast, ovarian and other cells from proliferating in an uncontrolled manner. If researchers find mutations, it’s relevant for a patient’s and family’s follow-up care — and that’s a prime example of how this newly designed app could play a life-saving role, she said.
Liz Burton was one of the first patients at the University of Pittsburgh to opt for the app -- called de-bi, which is short for decentralized biobank -- before undergoing a mastectomy for early-stage breast cancer in November, after it was diagnosed on a routine mammogram. She often takes part in medical research and looks forward to tracking her tissues.
“Anytime there’s a scientific experiment or study, I’m quick to participate -- to advance my own wellness as well as knowledge in general,” said Burton, 49, a life insurance service representative who lives in Carnegie, Pa. “It’s my way of contributing.”

Liz Burton was one of the first patients at the University of Pittsburgh to opt for the app before undergoing a mastectomy for early-stage breast cancer.
Liz Burton
The pilot program raises the issue of what investigators may owe study participants, especially since certain populations, such as Black and indigenous peoples, historically were not treated in an ethical manner for scientific purposes. “It’s a truly laudable effort,” Tamar Schiff, a postdoctoral fellow in medical ethics at New York University’s Grossman School of Medicine, said of the endeavor. “Research participants are beautifully altruistic.”
Lauren Sankary, a bioethicist and associate director of the neuroethics program at Cleveland Clinic, agrees that the pilot program provides increased transparency for study participants regarding how scientists use their tissues while acknowledging individuals’ contributions to research.
However, she added, “it may require researchers to develop a process for ongoing communication to be responsive to additional input from research participants.”
Peter H. Schwartz, professor of medicine and director of Indiana University’s Center for Bioethics in Indianapolis, said the program is promising, but he wonders what will happen if a patient has concerns about a particular research project involving their tissues.
“I can imagine a situation where a patient objects to their sample being used for some disease they’ve never heard about, or which carries some kind of stigma like a mental illness,” Schwartz said, noting that researchers would have to evaluate how to react. “There’s no simple answer to those questions, but the technology has to be assessed with an eye to the problems it could raise.”
To truly make a difference, blockchain must enable broad consent from patients, not just de-identification.
As a result, researchers may need to factor in how much information to share with patients and how to explain it, Schiff said. There are also concerns that in tracking their samples, patients could tell others what they learned before researchers are ready to publicly release this information. However, Bogdan, the vice president of the HIMSS nonprofit, believes only a minimal study identifier would be stored in an NFT, not patient data, research results or any type of proprietary trial information.
Some patients may be confused by blockchain and reluctant to embrace it. “The complexity of NFTs may prevent the average citizen from capitalizing on their potential or vendors willing to participate in the blockchain network,” Bogdan said. “Blockchain technology is also quite costly in terms of computational power and energy consumption, contributing to greenhouse gas emissions and climate change.”
In addition, this nascent, groundbreaking technology is immature and vulnerable to data security flaws, disputes over intellectual property rights and privacy issues, though it does offer baseline protections to maintain confidentiality. To truly make a difference, blockchain must enable broad consent from patients, not just de-identification, said Robyn Shapiro, a bioethicist and founding attorney at Health Sciences Law Group near Milwaukee.
The Henrietta Lacks story is a prime example, Shapiro noted. During her treatment for cervical cancer at Johns Hopkins, Lacks’s tissue was de-identified (albeit not entirely, because her cell line, HeLa, bore her initials). After her death, those cells were replicated and distributed for important and lucrative research and product development purposes without her knowledge or consent.
Nonetheless, Shapiro thinks that the initiative by the University of Pittsburgh and Johns Hopkins has potential to solve some ethical challenges involved in research use of biospecimens. “Compared to the system that allowed Lacks’s cells to be used without her permission, Shapiro said, “blockchain technology using nonfungible tokens that allow patients to follow their samples may enhance transparency, accountability and respect for persons who contribute their tissue and clinical data for research.”
Read more about laws that have prevented people from the rights to their own cells.

