This Resistance Fighter Invented Dialysis in Nazi-Occupied Holland
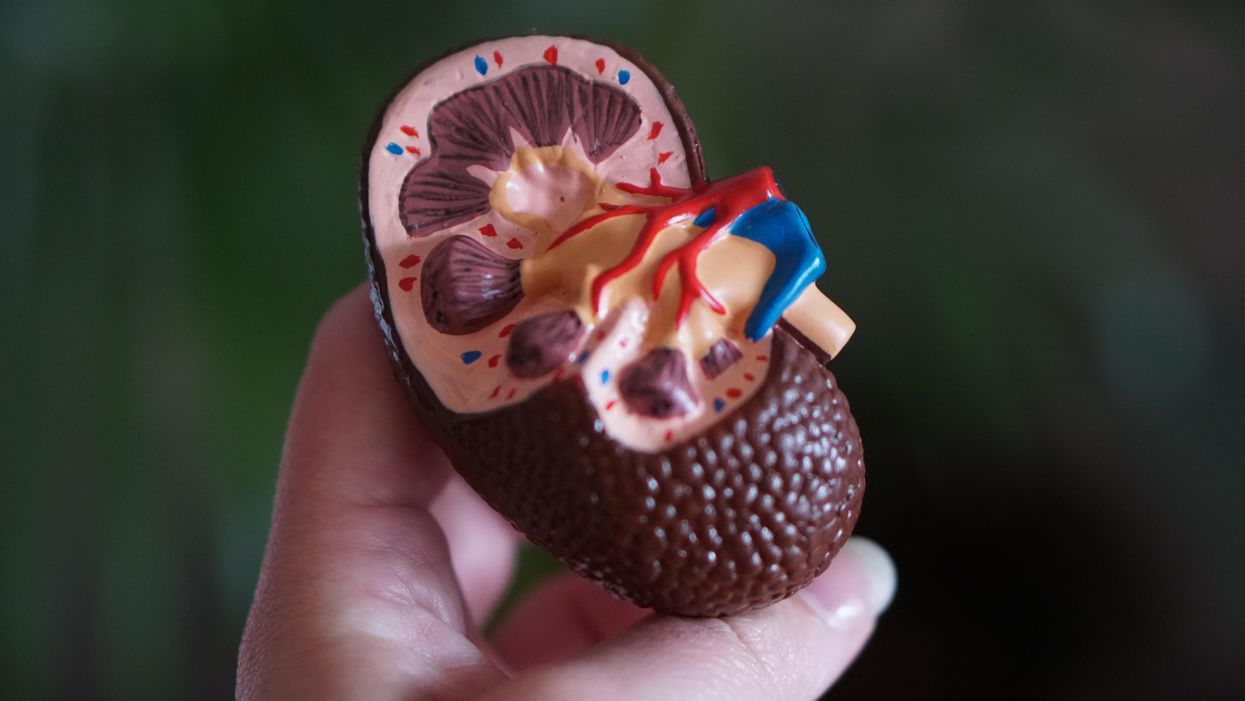
When Willem Johan Kolff invented dialysis, the "father" of artificial organs was just getting started.
One of the Netherlands’ most famous pieces of pop culture is “Soldier of Orange.” It’s the title of the country’s most celebrated war memoir, movie and epic stage musical, all of which detail the exploits of the nation’s resistance fighters during World War II.
Willem Johan Kolff was a member of the Dutch resistance, but he doesn’t rate a mention in the “Solider of Orange” canon. Yet his wartime toils in a rural backwater not only changed medicine, but the world.
Kolff had been a physician less than two years before Germany invaded the Netherlands in May 1940. He had been engaged in post-graduate studies at the University of Gronigen but withdrew because he refused to accommodate the demands of the Nazi occupiers. Kolff’s Jewish supervisor made an even starker choice: He committed suicide.
After his departure from the university, Kolff took a job managing a small hospital in Kampen. Located 50 miles from the heavily populated coastal region, the facility was far enough away from the prying eyes of Germans that not only could Kolff care for patients, he could hide fellow resistance fighters and even Jewish refugees in relative safety. Kolff coached many of them to feign convincing terminal illnesses so the Nazis would allow them to remain in the hospital.
Despite the demands of practicing medicine and resistance work, Kolff still found time to conduct research. He had been haunted and inspired when, not long before the Nazi invasion, one of his patients died in agony from kidney disease. Kolff wanted to find a way to save future patients.
He broke his problem down to a simple task: If he could remove 20 grams of urea from a patient’s blood in 24 hours, they would survive. He began experimenting with ways to filter blood and return it to a patient’s body. Since the war had ground all non-military manufacturing to a halt, he was mostly forced to make do with material he could find at the hospital and around Kampen. Kolff eventually built a device from a washing machine parts, juice cans, sausage casings, a valve from an old Ford automobile radiator, and even scrap from a downed German aircraft.
The world’s first dialysis machine was hardly imposing; it resembled a rotating drum for a bingo game or raffle. Yet it carried on the highly sophisticated task of moving a patient’s blood through a semi-permeable membrane (about a 50-foot length of sausage casings) into a saline solution that drew out urea while leaving the blood cells untouched.
In emigrating to the U.S. to practice medicine, Kolff's intent was twofold: Advocate for a wider adoption of dialysis, and work on new projects. He wildly succeeded at both.
Kolff began using the machine to treat patients in 1943, most of whom had lapsed into comas due to their kidney failure. But like most groundbreaking medical devices, it was not an immediate success. By the end of the war, Kolff had dialyzed more than a dozen patients, but all had died. He briefly suspended use of the device after the Allied invasion of Europe, but he continued to refine its operation and the administration of blood thinners to patients.
In September 1945, Kolff dialyzed another comatose patient, 67-year-old Sofia Maria Schafstadt. She regained consciousness after 11 hours, and would live well into the 1950s with Kolff’s assistance. Yet this triumph contained a dark irony: At the time of her treatment, Schafstadt had been imprisoned for collaborating with the Germans.
With a tattered Europe struggling to overcome the destruction of the war, Kolff and his family emigrated to the U.S. in 1950, where he began working for the Cleveland Clinic while undergoing the naturalization process so he could practice medicine in the U.S. His intent was twofold: Advocate for a wider adoption of dialysis, and work on new projects. He wildly succeeded at both.
By the mid-1950s, dialysis machines had become reliable and life-saving medical devices, and Kolff had become a U.S. citizen. About that time he invented a membrane oxygenator that could be used in heart bypass surgeries. This was a critical component of the heart-lung machine, which would make heart transplants possible and bypass surgeries routine. He also invented among the very first practical artificial hearts, which in 1957 kept a dog alive for 90 minutes.
Kolff moved to the University of Utah in 1967 to become director of its Institute for Biomedical Engineering. It was a promising time for such a move, as the first successful transplant of a donor heart to a human occurred that year. But he was interested in going a step further and creating an artificial heart for human use.
It took more than a decade of tinkering and research, but in 1982, a team of physicians and engineers led by Kolff succeeded in implanting the first artificial heart in dentist Barney Clark, whose failing health disqualified him from a heart transplant. Although Clark died in March 1983 after 112 days tethered to the device, that it kept him alive generated international headlines. While graduate student Robert Jarvik received the named credit for the heart, he was directly supervised by Kolff, whose various endeavors into artificial organ research at the University of Utah were segmented into numerous teams.
Forty years later, several artificial hearts have been approved for use by the Food and Drug Administration, although all are a “bridge” that allow patients to wait for a transplant.
Kolff continued researching and tinkering with biomedical devices – including artificial eyes and ears – until he retired in 1997 at the age of 86. When he died in 2009, the medical community acknowledged that he was not only a pioneer in biotechnology, but the “father” of artificial organs.
Air pollution can lead to lung cancer. The connection suggests new ways to stop cancer in its tracks.
Researchers at Francis Crick Institute found that air pollution can "wake up" existing mutations, triggering them to turn into lung cancer. The scientists used their new understanding about this connection to prevent cancer in mice.
Forget taking a deep breath. Around the world, 99 percent of people breathe air polluted to unsafe levels, according to data from the World Health Organization. Activities such as burning fossil fuels release greenhouse gases that contribute to air pollution, which could lead to heart disease, stroke, asthma, emphysema, and some types of cancer.
“The burden of disease attributable to air pollution is now estimated to be on a par with other major global health risks such as unhealthy diet and tobacco smoking, and air pollution is now recognized as the single biggest environmental threat to human health,” wrote the authors of a 2021 WHO report.
The majority of lung cancer is attributed to smoking. But as pollution levels have increased, and anti-smoking campaigns have discouraged smoking, the proportion of lung cancers diagnosed in non-smokers has grown. The CDC estimates that 10 to 20 percent of lung cancers in the U.S. currently occur in non-smokers.
The mechanism between air pollution and the development of lung cancer has been unclear, but researchers at London’s Francis Crick Institute recently made an important breakthrough in understanding the connection. Lead investigator Charles Swanton presented this research last month at a conference in Paris.
Pollution awakens mutations
The Crick Institute scientists were able to identify a new link between common air pollutants and non-small cell lung cancer (NSCLC). They focused on pollutants called particulate matter, or PM, that are 2.5 microns wide, narrower than human cells.
Most cancer diagnosed in non-smoking people is NSCLC, but this type of cancer hasn’t received the same research attention as more common lung cancers found in smokers, according to Clare Weeden, a cancer researcher at the Crick Institute and a co-author of the study.
“This is a really underserved and under-researched population that we really need to tackle, as well as lung cancers that occur in smokers,” she says. “Lung cancer is the number one cancer killer worldwide.”
In the past, some researchers believed air pollution caused mutations that led to cancer. Others believed these mutations could remain dormant without any detriment to health until pollutants or other stressors triggered them to become cancerous. Reviving the latter hypothesis that carcinogens may activate pre-existing mutations, instead of directly causing them, the Crick researchers analyzed samples from 463,679 people in the UK and parts of Asia, noting mutations and comparing changes in gene expression in mice and human cells.
“The mutation can exist in a nascent clone without causing cancer,” says Emilia Lim, a bioinformatics expert and a co-first author of the Crick study. “It is the carcinogen that promotes a conducive environment for this one little clone to grow and expand into cancer. Through our work, we were able to revive excitement for this hypothesis and bring it to light.”
The study explains a confusing pattern of lung cancer developing, particularly in women, despite a lack of environmental risk factors like smoking, secondhand smoke, or radon exposure. The culprit in these cases may have been too much PM 2.5 exposure.
Other researchers had previously identified a link between mutations in certain genes that control epidermal growth factor receptors, or EGFR mutations, and the development of NSCLC. In a 2019 study of 250 people with this type of cancer, about 32 percent had the mutation. Women are more likely to have EGFR mutations than men.
Not everyone who has the EGFR mutation will develop lung cancer. Respirologist Stephen Lam studies lung cancer at the BC Cancer Research Centre in Vancouver, Canada, but was not involved in the Crick Institute research. He says the study explains a confusing pattern of lung cancer developing, particularly in women, despite a lack of environmental risk factors like smoking, secondhand smoke, or radon exposure. The culprit in these cases may have been too much PM 2.5 exposure.
More exposure leads to inflammation and lesions
The Crick researchers found that an excess of PM 2.5 in the air sparked an inflammatory process in cells within the lung. This inflammation set the stage for NSCLC to develop in people and mice with existing EGFR mutations.
The researchers also exposed mice without EGFR mutations to PM 2.5 pollution—an experiment that couldn’t be ethically conducted in humans—to link pollution exposure to NSCLC. The mice experiments also showed that NSCLC is dose-dependent; higher levels of exposure were associated with higher number of cancerous lesions forming.
Ultimately, the study “fundamentally changed how we view lung cancer in people who have never smoked,” said Swanton in a Crick Institute press release. “Cells with cancer-causing mutations accumulate naturally as we age, but they are normally inactive. We’ve demonstrated that air pollution wakes these cells up in the lungs, encouraging them to grow and potentially form tumors.”
Preventing cancer before it begins
Targeted therapies already exist for people with EGFR mutations who’ve developed NSCLC, but they have many side effects, according to Weeden. Researchers hope that making more definitive links between pollutants and cancer could help prevent people with EGFR or other mutations from developing lung cancer in the first place.
Along those lines, as an additional component of their study presented last month, the Crick researchers were able to prevent cancer in mice that had the EGFR mutations by blocking inflammation. They used an antibody to inhibit a protein called interleukin 1 beta, which plays a key role in inflammation. Scientists could eventually use such antibodies or other therapies to make a drug treatment that people can take to stop cancer in its tracks, even if they live in highly polluted areas.
Such potential could reach beyond lung cancer; in the past, Crick and other researchers have also found associations between exposure to air pollution and mesothelioma, as well as cancers of the small intestine, lip, mouth, and throat. These links could be meaningful to a growing number of people as climate change intensifies, and with increases in air pollution from fossil fuel combustion and natural disasters like forest fires.
Plus, air pollution is just one external condition that can flip the switch of these inflammatory pathways. Identifying a link between pollution and cancer “has wide ramifications for many other environmental factors that may [play] similar roles,” Weedon says. She hopes that the Crick study and future research in this area will offer some hope for non-smokers frustrated by cancer diagnoses.
Naked Mole Rats Defy Aging. One Scientist Has Dedicated Her Career to Finding Out How.
Naked mole rats have extraordinarily long lifespans and are extremely resistant to cancer.
Rochelle "Shelley" Buffenstein has one of the world's largest, if not the largest, lab-dwelling colonies of the naked mole rat. (No one has done a worldwide tabulation, but she has 4,500 of them.) Buffenstein has spent decades studying the little subterranean-dwelling rodents. Over the years, she and her colleagues have uncovered one surprising discovery after another, which has led them to re-orient the whole field of anti-aging research.
Naked mole rats defy everything we thought we knew about aging. These strange little rodents from arid regions of Africa, such as Kenya, Ethiopia and Somalia, live up to ten times longer than their size would suggest. And unlike virtually every other animal, they don't lose physical or cognitive abilities with age, and even retain their fertility up until the end of life. They appear to have active defenses against the ravages of time, suggesting that aging may not be inevitable. Could these unusual creatures teach humans how to extend life and ameliorate aging?
Buffenstein, who is senior principle investigator at Calico Life Sciences, has dedicated her life to finding out. Her early interest in the animals of what is now Zimbabwe led to her current position as a cutting-edge anti-aging researcher at Calico, the Google-funded health venture launched in 2013. The notoriously secretive company is focused on untangling the mysteries of why animals and people age, and whether there are ways to slow or temporarily arrest the process.
The small, wrinkly animal, which lives in underground burrows in the hot, arid regions of Africa, is hardly the beauty queen of the mammalian kingdom. Furless, buck-toothed and tiny-eyed, the creatures look like they could use a good orthodontist, a protective suit of clothes and possibly, some spectacles to enhance their eyesight. But these rats more than make up for their unimpressive looks with their superlative ability to adapt to some of the most inhospitable conditions on earth.
Based on the usual rule that body size predicts lifespan, naked mole rats shouldn't live that long. After all, similarly-sized rodents like mice have a life expectancy of two years or less. But Buffenstein was one of the first scientists to recognize that naked mole rats live an extraordinarily long time, with her oldest animal approaching 39 years of age. In addition, they never become geriatric in the human sense, defying the common signs of aging — age-related diseases, cognitive decline and even menopause. In fact, the queens, or females that do all the breeding in a bee-like underground colony, remain fertile and give birth to healthy pups up until what would be considered very old age in humans. And the naked mole rat has other curious abilities, such as the ability to endure extreme low-oxygen, or hypoxic, conditions like those they encounter in their underground nests.
"One thing we've learned from these animals is that they stay healthy until the very end."
It's not that the naked mole rat isn't subject to the vicissitudes of life, or the normal wear and tear of biological processes. Over the years, Buffenstein and her colleagues have discovered that, while the process of oxidative stress — thought for 50 years to be the main cause of aging — occurs in the naked mole rat just as in any other animal, its damage does not accumulate with age. Oxidative stress occurs during normal cell metabolism when oxygen "free radicals" with one or more unpaired electrons wreak havoc on large cellular molecules, leaving microscopic debris in their wake that clogs up the gears of healthy cell function. Somehow, naked mole rats have an enhanced ability to clear out the damaged cells and molecules before they can set off the usual chain reaction of cell dysfunction and death, according to a 2013 paper in which Buffenstein is the lead author.
Oxidative stress is not the only factor known to be problematic in aging. Slowly accumulating damage to DNA typically leads to protein malfunction and improper folding. In humans and most other animals, these protein fragments can accumulate in cells and gum up the works. Only not so much in naked mole rats, which are able to maintain normal protein folding throughout their long life. After years of discoveries like these, Buffenstein has gradually reframed her focus from "what goes wrong to produce aging?" to "what goes right in the naked mole rat to help it defy the normal wear and tear of life?" Buffenstein's research suggests that the tiny mammals have a unique ability to somehow clear out damaged protein fragments and other toxic debris before they can cause disease and aging.
How She Got Here
Buffenstein ascribes her initial acquaintance with the naked mole rat to serendipity. Back in 1979, her postgraduate mentor Jenny Jarvis at the University of Cape Town in South Africa kept a small colony of rats in her office while studying the mechanisms that lead to the animals' unusual adaptive capabilities. It was Buffenstein's job to take care of them. Working with Jarvis, Buffenstein focused on understanding their unique adaptations to the extreme conditions of their natural habitat.
They studied the unusual behaviors regulating the rat colonies. For instance, they observed that designated "workers" dig the entire colony's underground tunnels and a single reproducing female breeds with only a small number of males. Buffenstein also examined how these animals are able to survive without the "sunshine hormone" — vitamin D — and their unusual modes of regulating their internal temperatures and converting food into energy. Though classified as mammals, the rodents simply don't conform to the mammalian handbook, having found ingenuous ways to alter their bodies and behavior that is fine-tuned to the scorching heat and aridity of their environment.
To escape the heat, they simply burrow underground and live in elaborate tunnels. To cope with the low-oxygen conditions underground, they slowed their metabolism and learned to live for extended periods of time in such hypoxic conditions that an ordinary animal would quickly suffocate. But it was slowly dawning on Buffenstein that the small creatures were exceptional in additional ways.
When Buffenstein got her first academic position at the University of Witwatersrand in Johannesburg, Jarvis said she could take some of the naked mole rats with her. When she did, Buffenstein noticed that the animals were living far longer than similarly sized rodents. "At that stage, they were about ten years old. Little did I know how long they would eventually show us they could live," she says.
In 1997, after accepting a position at the City College of New York, Buffenstein moved to the U.S. and took her rat colony with her. There she was able to pursue an evolving narrative about the humble naked mole rat that continued to defy expectations. As the years passed, it was becoming more and more evident that her observations could have major implications for aging research. Eventually, she took a position at the Barshop Institute for Aging and Longevity Studies in San Antonio, Texas.
One early observation of Buffenstein's suggested that the species most often used in aging research—mice, roundworms, fruit flies and yeast—have short lifespans and poor defenses against aging. These animals provide important insights into how aging works, and have revealed possible targets for intervention. But they don't show what goes right in apparently non-aging animals like the naked mole rat.
Buffenstein's years of studying the rats has laid the foundation for a whole new perspective in aging research.
"My hypothesis," she says, "is that naked mole rats are very good at removing damaged macromolecules and cells, thereby maintaining homeostasis and cell and tissue function. All the repair pathways examined by us and others in the field point to more efficient repair and more rapid responses to damaging agents." These include things like free radicals and radiation.
Buffenstein’s Legacy
Some researchers today are building on Buffenstein's foundational discoveries to home in on possible anti-aging mechanisms that lead to the extraordinary resilience of naked mole rats. University of Cambridge researcher and co-founder of the institution's Naked Mole-Rat Initiative, Ewan St. John Smith, is studying the animal's resistance to cancer.
In a 2020 paper published in Nature, Smith and his colleagues established that naked mole rats harbor cancer-causing genes, and these genes occasionally create cancer cells. But something in the rats shuts the multiplication process down before the cells can grow out of control and form tumors. Now, scientists want to know what mechanisms, exactly, are at play in preventing the cells from invading healthy tissues. Smith has hypothesized that the answer is somehow embedded in interactions in the cells' microenvironment.
He also thinks the animal's immune system could just be very effective at seeking out and destroying cancer cells. Several current cancer therapies work by boosting the body's immune system so it can attack and eliminate the toxic cells. It's possible that the naked mole rat's immune system naturally goes into hyper-drive when cancer cells appear, enabling it to nip the disease in the bud before tumors can form. A pharmacologist by training, Smith thinks that if there is some chemical mediator in the naked mole rat that supercharges its immune cells, perhaps that mediator can be synthesized in a drug to treat humans for cancer.
The naked mole rat's extreme tolerance to hypoxia could also play a role. "Interestingly," he says, "when cells become cancerous, they also become hypoxic, and naked mole rats are known to be very resistant to hypoxia.
He notes that a form of low-level hypoxia is also present in the bodies and brains of both aged mice and older humans. It's commonly seen in the brains of humans with Alzheimer's disease and other forms of age-related dementia. This suggests that hypoxia in humans — and in other mammals — may have a role to play in Alzheimer's and the aging process itself. Resistance to hypoxia could be why the naked mole rat, in Smith's words, "chugs along quite happily" in conditions that in humans are associated with disease and decline.
Smith cheerfully acknowledges his debt to Buffenstein for laying so much of the groundwork in a field rife with possible implications for anti-aging. "Shelley is amazing," he says. "Naked mole rats have a queen and I always refer to her as the queen of the naked mole rat world." In fact, Buffenstein gave Smith his first colony of rats, which he's since grown to about 150. "Some of them will still be around when I retire," he jokes.
Vera Gorbunova, a professor of biology and oncology at the University of Rochester who studies both longevity and cancer in naked mole rats, credits Buffenstein with getting others to study the animals for anti-aging purposes. Gorbunova believes that "cancer and aging go hand-in-hand" and that longer-lived animals have better, more accurate DNA repair.
Gorbunova is especially interested in the naked mole rat's ability to secrete a superabundance of a "super-sugar" molecule called hyaluronan, a ubiquitous additive to skin creams for its moisturizing effect. Gorbunova and others have observed that the presence of high concentrations of hyaluronan in the naked mole rat's extracellular matrix — the chemical-rich solution between cells — prevents the overcrowding of cells. This, perhaps, could be the key to the animal's ability to stop tumors from forming.
Hyaluronan is also present in the extracellular matrix of humans, but the naked mole rat molecule is more than five times larger than the versions found in humans or mice, and is thought to play a significant part in the animal's DNA repair. But just rubbing a cream containing hyaluronan over your skin won't stop cancer or aging. High concentrations of the substance in the extracellular matrix throughout your body would likely be needed.
Gorbunova notes that the naked mole rat offers a multitude of possibilities that could eventually lead to drugs to slow human aging. "I'm optimistic that there are many different strategies, because the naked mole rat likely has many processes going on that fight aging," she says. "I think that in a relatively short time, there will be bonafide treatments to test in animals. One thing we've learned from these animals is that they stay healthy until the very end."
So if naked mole rats don't become frail with age or develop age-related diseases, what does kill them? The answer, unfortunately, is usually other naked mole rats. Buffenstein has long noted that even though they live in highly cooperative colonies, they can be quite cantankerous when there's a disruption in the hierarchy, a sentiment echoed by Gorbunova. "Sometimes there are periods of peace and quiet, but if something happens to the queen, all hell breaks loose," she says. "If the queen is strong, everybody knows their place," but if the queen dies, the new queen is inevitably decided by violent competition.
To the casual observer, a strange, wrinkly rodent like the naked mole rat might seem to have little to teach us about ourselves, but Buffenstein is confident that her discoveries could have major implications for human longevity research. Today, at Calico's labs in San Francisco, she's focused entirely on the determining how anti-aging defense mechanisms in the rats could lead to similar defenses being stimulated or introduced in humans.
"The million-dollar question is, what are the mechanisms protecting against aging, and can these be translated into therapies to delay or abrogate human aging, too?"
Buffenstein fired up a new generation of scientists with multiple discoveries, especially the fundamental one that naked mole rats are subject to the same wear and tear over time as the rest of us, but somehow manage to reverse it. These days, the trailblazer is at work on untangling the molecular mechanisms involved in the animal's resistance to cardiac aging. On top of everything else, the small creature has a unique ability to fight off the scourge of heart disease, which is the leading cause of death in the industrialized world.
After all, the point is not to extend old age, but to slow down aging itself so that frailty and disability are compressed into a brief period after a long-extended period of vitality. By switching the focus from what goes wrong to mechanisms that defend against aging in the first place, the discoveries of Buffenstein and a new generation of researchers who are building on her groundbreaking research promise to be a driving force in the quest to extend not only life, but healthy, vigorous life in humans.
This article was first published by Leaps.org on June 23, 2021.

