Scientists are making machines, wearable and implantable, to act as kidneys
Recent advancements in engineering mean that the first preclinical trials for an artificial kidney could happen soon.
Like all those whose kidneys have failed, Scott Burton’s life revolves around dialysis. For nearly two decades, Burton has been hooked up (or, since 2020, has hooked himself up at home) to a dialysis machine that performs the job his kidneys normally would. The process is arduous, time-consuming, and expensive. Except for a brief window before his body rejected a kidney transplant, Burton has depended on machines to take the place of his kidneys since he was 12-years-old. His whole life, the 39-year-old says, revolves around dialysis.
“Whenever I try to plan anything, I also have to plan my dialysis,” says Burton says, who works as a freelance videographer and editor. “It’s a full-time job in itself.”
Many of those on dialysis are in line for a kidney transplant that would allow them to trade thrice-weekly dialysis and strict dietary limits for a lifetime of immunosuppressants. Burton’s previous transplant means that his body will likely reject another donated kidney unless it matches perfectly—something he’s not counting on. It’s why he’s enthusiastic about the development of artificial kidneys, small wearable or implantable devices that would do the job of a healthy kidney while giving users like Burton more flexibility for traveling, working, and more.
Still, the devices aren’t ready for testing in humans—yet. But recent advancements in engineering mean that the first preclinical trials for an artificial kidney could happen soon, according to Jonathan Himmelfarb, a nephrologist at the University of Washington.
“It would liberate people with kidney failure,” Himmelfarb says.
An engineering marvel
Compared to the heart or the brain, the kidney doesn’t get as much respect from the medical profession, but its job is far more complex. “It does hundreds of different things,” says UCLA’s Ira Kurtz.
Kurtz would know. He’s worked as a nephrologist for 37 years, devoting his career to helping those with kidney disease. While his colleagues in cardiology and endocrinology have seen major advances in the development of artificial hearts and insulin pumps, little has changed for patients on hemodialysis. The machines remain bulky and require large volumes of a liquid called dialysate to remove toxins from a patient’s blood, along with gallons of purified water. A kidney transplant is the next best thing to someone’s own, functioning organ, but with over 600,000 Americans on dialysis and only about 100,000 kidney transplants each year, most of those in kidney failure are stuck on dialysis.
Part of the lack of progress in artificial kidney design is the sheer complexity of the kidney’s job. Each of the 45 different cell types in the kidney do something different.
Part of the lack of progress in artificial kidney design is the sheer complexity of the kidney’s job. To build an artificial heart, Kurtz says, you basically need to engineer a pump. An artificial pancreas needs to balance blood sugar levels with insulin secretion. While neither of these tasks is simple, they are fairly straightforward. The kidney, on the other hand, does more than get rid of waste products like urea and other toxins. Each of the 45 different cell types in the kidney do something different, helping to regulate electrolytes like sodium, potassium, and phosphorous; maintaining blood pressure and water balance; guiding the body’s hormonal and inflammatory responses; and aiding in the formation of red blood cells.

There's been little progress for patients during Ira Kurtz's 37 years as a nephrologist. Artificial kidneys would change that.
UCLA
Dialysis primarily filters waste, and does so well enough to keep someone alive, but it isn’t a true artificial kidney because it doesn’t perform the kidney’s other jobs, according to Kurtz, such as sensing levels of toxins, wastes, and electrolytes in the blood. Due to the size and water requirements of existing dialysis machines, the equipment isn’t portable. Physicians write a prescription for a certain duration of dialysis and assess how well it’s working with semi-regular blood tests. The process of dialysis itself, however, is conducted blind. Doctors can’t tell how much dialysis a patient needs based on kidney values at the time of treatment, says Meera Harhay, a nephrologist at Drexel University in Philadelphia.
But it’s the impact of dialysis on their day-to-day lives that creates the most problems for patients. Only one-quarter of those on dialysis are able to remain employed (compared to 85% of similar-aged adults), and many report a low quality of life. Having more flexibility in life would make a major different to her patients, Harhay says.
“Almost half their week is taken up by the burden of their treatment. It really eats away at their freedom and their ability to do things that add value to their life,” she says.
Art imitates life
The challenge for artificial kidney designers was how to compress the kidney’s natural functions into a portable, wearable, or implantable device that wouldn’t need constant access to gallons of purified and sterilized water. The other universal challenge they faced was ensuring that any part of the artificial kidney that would come in contact with blood was kept germ-free to prevent infection.
As part of the 2021 KidneyX Prize, a partnership between the U.S. Department of Health and Human Services and the American Society of Nephrology, inventors were challenged to create prototypes for artificial kidneys. Himmelfarb’s team at the University of Washington’s Center for Dialysis Innovation won the prize by focusing on miniaturizing existing technologies to create a portable dialysis machine. The backpack sized AKTIV device (Ambulatory Kidney to Increase Vitality) will recycle dialysate in a closed loop system that removes urea from blood and uses light-based chemical reactions to convert the urea to nitrogen and carbon dioxide, which allows the dialysate to be recirculated.
Himmelfarb says that the AKTIV can be used when at home, work, or traveling, which will give users more flexibility and freedom. “If you had a 30-pound device that you could put in the overhead bins when traveling, you could go visit your grandkids,” he says.
Kurtz’s team at UCLA partnered with the U.S. Kidney Research Corporation and Arkansas University to develop a dialysate-free desktop device (about the size of a small printer) as the first phase of a progression that will he hopes will lead to something small and implantable. Part of the reason for the artificial kidney’s size, Kurtz says, is the number of functions his team are cramming into it. Not only will it filter urea from blood, but it will also use electricity to help regulate electrolyte levels in a process called electrodeionization. Kurtz emphasizes that these additional functions are what makes his design a true artificial kidney instead of just a small dialysis machine.
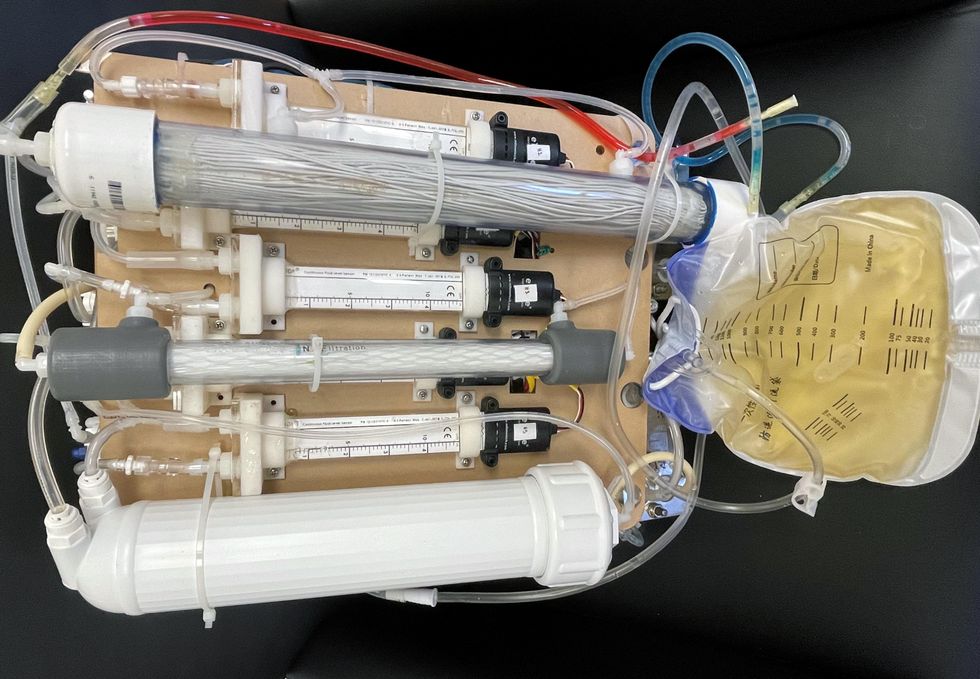
One version of an artificial kidney.
UCLA
“It doesn't have just a static function. It has a bank of sensors that measure chemicals in the blood and feeds that information back to the device,” Kurtz says.
Other startups are getting in on the game. Nephria Bio, a spinout from the South Korean-based EOFlow, is working to develop a wearable dialysis device, akin to an insulin pump, that uses miniature cartridges with nanomaterial filters to clean blood (Harhay is a scientific advisor to Nephria). Ian Welsford, Nephria’s co-founder and CTO, says that the device’s design means that it can also be used to treat acute kidney injuries in resource-limited settings. These potentials have garnered interest and investment in artificial kidneys from the U.S. Department of Defense.
For his part, Burton is most interested in an implantable device, as that would give him the most freedom. Even having a regular outpatient procedure to change batteries or filters would be a minor inconvenience to him.
“Being plugged into a machine, that’s not mimicking life,” he says.
This article was first published by Leaps.org on May 5, 2022.
Leading XPRIZE Healthspan and Beating Negativity with Dr. Peter Diamandis
XPRIZE founder and chairman Peter Diamandis launches XPRIZE Healthspan at an event on November 29.
A new competition by the XPRIZE Foundation is offering $101 million to researchers who discover therapies that give a boost to people aged 65-80 so their bodies perform more like when they were middle-aged.
For today’s podcast episode, I talked with Dr. Peter Diamandis, XPRIZE’s founder and executive chairman. Under Peter’s leadership, XPRIZE has launched 27 previous competitions with over $300 million in prize purses. The latest contest aims to enhance healthspan, or the period of life when older people can play with their grandkids without any restriction, disability or disease. Such breakthroughs could help prevent chronic diseases that are closely linked to aging. These illnesses are costly to manage and threaten to overwhelm the healthcare system, as the number of Americans over age 65 is rising fast.
In this competition, called XPRIZE Healthspan, multiple awards are available, depending on what’s achieved, with support from the nonprofit Hevolution Foundation and Chip Wilson, the founder of Lululemon and nonprofit SOLVE FSHD. The biggest prize, $81 million, is for improvements in cognition, muscle and immunity by 20 years. An improvement of 15 years will net $71 million, and 10 years will net $61 million.
In our conversation for this episode, Peter talks about his plans for XPRIZE Healthspan and why exponential technologies make the current era - even with all of its challenges - the most exciting time in human history. We discuss the best mental outlook that supports a person in becoming truly innovative, as well as the downsides of too much risk aversion. We talk about how to overcome the negativity bias in ourselves and in mainstream media, how Peter has shifted his own mindset to become more positive over the years, how to inspire a culture of innovation, Peter’s personal recommendations for lifestyle strategies to live longer and healthier, the innovations we can expect in various fields by 2030, the future of education and the importance of democratizing tech and innovation.
In addition to Peter’s pioneering leadership of XPRIZE, he is also the Executive Founder of Singularity University. In 2014, he was named by Fortune as one of the “World’s 50 Greatest Leaders.” As an entrepreneur, he’s started over 25 companies in the areas of health-tech, space, venture capital and education. He’s Co-founder and Vice-Chairman of two public companies, Celularity and Vaxxinity, plus being Co-founder & Chairman of Fountain Life, a fully-integrated platform delivering predictive, preventative, personalized and data-driven health. He also serves as Co-founder of BOLD Capital Partners, a venture fund with a half-billion dollars under management being invested in exponential technologies and longevity companies. Peter is a New York Times Bestselling author of four books, noted during our conversation and in the show notes of this episode. He has degrees in molecular genetics and aerospace engineering from MIT and holds an M.D. from Harvard Medical School.
Show links
- Peter Diamandis bio
- New XPRIZE Healthspan
- Peter Diamandis books
- 27 XPRIZE competitions and counting
- Life Force by Peter Diamandis and Tony Robbins
- Peter Diamandis Twitter
- Longevity Insider newsletter – AI identifies the news
- Peter Diamandis Longevity Handbook
- Hevolution funding for longevity

XPRIZE Founder Peter Diamandis speaks with Mehmoud Khan, CEO of Hevolution Foundation, at the launch of XPRIZE Healthspan.
Hevolution Foundation
Important findings are starting to emerge from research on how genes shape the human response to the Covid virus.
From infections with no symptoms to why men are more likely to be hospitalized in the ICU and die of COVID-19, new research shows that your genes play a significant role
Early in the pandemic, genetic research focused on the virus because it was readily available. Plus, the virus contains only 30,000 bases in a dozen functional genes, so it's relatively easy and affordable to sequence. Additionally, the rapid mutation of the virus and its ability to escape antibody control fueled waves of different variants and provided a reason to follow viral genetics.
In comparison, there are many more genes of the human immune system and cellular functions that affect viral replication, with about 3.2 billion base pairs. Human studies require samples from large numbers of people, the analysis of each sample is vastly more complex, and sophisticated computer analysis often is required to make sense of the raw data. All of this takes time and large amounts of money, but important findings are beginning to emerge.
Asymptomatics
About half the people exposed to SARS-CoV-2, the virus that causes the COVID-19 disease, never develop symptoms of this disease, or their symptoms are so mild they often go unnoticed. One piece of understanding the phenomena came when researchers showed that exposure to OC43, a common coronavirus that results in symptoms of a cold, generates immune system T cells that also help protect against SARS-CoV-2.
Jill Hollenbach, an immunologist at the University of California at San Francisco, sought to identify the gene behind that immune protection. Most COVID-19 genetic studies are done with the most seriously ill patients because they are hospitalized and thus available. “But 99 percent of people who get it will never see the inside of a hospital for COVID-19,” she says. “They are home, they are not interacting with the health care system.”
Early in the pandemic, when most labs were shut down, she tapped into the National Bone Marrow Donor Program database. It contains detailed information on donor human leukocyte antigens (HLAs), key genes in the immune system that must match up between donor and recipient for successful transplants of marrow or organs. Each HLA can contain alleles, slight molecular differences in the DNA of the HLA, which can affect its function. Potential HLA combinations can number in the tens of thousands across the world, says Hollenbach, but each person has a smaller number of those possible variants.
She teamed up with the COVID-19 Citizen Science Study a smartphone-based study to track COVID-19 symptoms and outcomes, to ask persons in the bone marrow donor registry about COVID-19. The study enlisted more than 30,000 volunteers. Those volunteers already had their HLAs annotated by the registry, and 1,428 tested positive for the virus.
Analyzing five key HLAs, she found an allele in the gene HLA-B*15:01 that was significantly overrepresented in people who didn’t have any symptoms. The effect was even stronger if a person had inherited the allele from both parents; these persons were “more than eight times more likely to remain asymptomatic than persons who did not carry the genetic variant,” she says. Altogether this HLA was present in about 10 percent of the general European population but double that percentage in the asymptomatic group. Hollenbach and her colleagues were able confirm this in other different groups of patients.
What made the allele so potent against SARS-CoV-2? Part of the answer came from x-ray crystallography. A key element was the molecular shape of parts of the cold virus OC43 and SARS-CoV-2. They were virtually identical, and the allele could bind very tightly to them, present their molecular antigens to T cells, and generate an extremely potent T cell response to the viruses. And “for whatever reasons that generated a lot of memory T cells that are going to stick around for a long time,” says Hollenbach. “This T cell response is very early in infection and ramps up very quickly, even before the antibody response.”
Understanding the genetics of the immune response to SARS-CoV-2 is important because it provides clues into the conditions of T cells and antigens that support a response without any symptoms, she says. “It gives us an opportunity to think about whether this might be a vaccine design strategy.”
Dead men
A researcher at the Leibniz Institute of Virology in Hamburg Germany, Guelsah Gabriel, was drawn to a question at the other end of the COVID-19 spectrum: why men more likely to be hospitalized and die from the infection. It wasn't that men were any more likely to be exposed to the virus but more likely, how their immune system reacted to it
Several studies had noted that testosterone levels were significantly lower in men hospitalized with COVID-19. And, in general, the lower the testosterone, the worse the prognosis. A year after recovery, about 30 percent of men still had lower than normal levels of testosterone, a condition known as hypogonadism. Most of the men also had elevated levels of estradiol, a female hormone (https://pubmed.ncbi.nlm.nih.gov/34402750/).
Every cell has a sex, expressing receptors for male and female hormones on their surface. Hormones docking with these receptors affect the cells' internal function and the signals they send to other cells. The number and role of these receptors varies from tissue to tissue.
Gabriel began her search by examining whole exome sequences, the protein-coding part of the genome, for key enzymes involved in the metabolism of sex hormones. The research team quickly zeroed in on CYP19A1, an enzyme that converts testosterone to estradiol. The gene that produces this enzyme has a number of different alleles, the molecular variants that affect the enzyme's rate of metabolizing the sex hormones. One genetic variant, CYP19A1 (Thr201Met), is typically found in 6.2 percent of all people, both men and women, but remarkably, they found it in 68.7 percent of men who were hospitalized with COVID-19.
Lung surprise
Lungs are the tissue most affected in COVID-19 disease. Gabriel wondered if the virus might be affecting expression of their target gene in the lung so that it produces more of the enzyme that converts testosterone to estradiol. Studying cells in a petri dish, they saw no change in gene expression when they infected cells of lung tissue with influenza and the original SARS-CoV viruses that caused the SARS outbreak in 2002. But exposure to SARS-CoV-2, the virus responsible for COVID-19, increased gene expression up to 40-fold, Gabriel says.
Did the same thing happen in humans? Autopsy examination of patients in three different cites found that “CYP19A1 was abundantly expressed in the lungs of COVID-19 males but not those who died of other respiratory infections,” says Gabriel. This increased enzyme production led likely to higher levels of estradiol in the lungs of men, which “is highly inflammatory, damages the tissue, and can result in fibrosis or scarring that inhibits lung function and repair long after the virus itself has disappeared.” Somehow the virus had acquired the capacity to upregulate expression of CYP19A1.
Only two COVID-19 positive females showed increased expression of this gene. The menopause status of these women, or whether they were on hormone replacement therapy was not known. That could be important because female hormones have a protective effect for cardiovascular disease, which women often lose after going through menopause, especially if they don’t start hormone replacement therapy. That sex-specific protection might also extend to COVID-19 and merits further study.
The team was able to confirm their findings in golden hamsters, the animal model of choice for studying COVID-19. Testosterone levels in male animals dropped 5-fold three days after infection and began to recover as viral levels declined. CYP19A1 transcription increased up to 15-fold in the lungs of the male but not the females. The study authors wrote, “Virus replication in the male lungs was negatively associated with testosterone levels.”
The medical community studying COVID-19 has slowly come to recognize the importance of adipose tissue, or fat cells. They are known to express abundant levels of CYP19A1 and play a significant role as metabolic tissue in COVID-19. Gabriel adds, “One of the key findings of our study is that upon SARS-CoV-2 infection, the lung suddenly turns into a metabolic organ by highly expressing” CYP19A1.
She also found evidence that SARS-CoV-2 can infect the gonads of hamsters, thereby likely depressing circulating levels of sex hormones. The researchers did not have autopsy samples to confirm this in humans, but others have shown that the virus can replicate in those tissues.
A possible treatment
Back in the lab, substituting low and high doses of testosterone in SARS-COV-2 infected male hamsters had opposite effects depending on testosterone dosage used. Gabriel says that hormone levels can vary so much, depending on health status and age and even may change throughout the day, that “it probably is much better to inhibit the enzyme” produced by CYP19A1 than try to balance the hormones.
Results were better with letrozole, a drug approved to treat hypogonadism in males, which reduces estradiol levels. The drug also showed benefit in male hamsters in terms of less severe disease and faster recovery. She says more details need to be worked out in using letrozole to treat COVID-19, but they are talking with hospitals about clinical trials of the drug.
Gabriel has proposed a four hit explanation of how COVID-19 can be so deadly for men: the metabolic quartet. First is the genetic risk factor of CYP19A1 (Thr201Met), then comes SARS-CoV-2 infection that induces even greater expression of this gene and the deleterious increase of estradiol in the lung. Age-related hypogonadism and the heightened inflammation of obesity, known to affect CYP19A1 activity, are contributing factors in this deadly perfect storm of events.
Studying host genetics, says Gabriel, can reveal new mechanisms that yield promising avenues for further study. It’s also uniting different fields of science into a new, collaborative approach they’re calling “infection endocrinology,” she says.