Scientists are making machines, wearable and implantable, to act as kidneys
Recent advancements in engineering mean that the first preclinical trials for an artificial kidney could happen as soon as 18 months from now
Like all those whose kidneys have failed, Scott Burton’s life revolves around dialysis. For nearly two decades, Burton has been hooked up (or, since 2020, has hooked himself up at home) to a dialysis machine that performs the job his kidneys normally would. The process is arduous, time-consuming, and expensive. Except for a brief window before his body rejected a kidney transplant, Burton has depended on machines to take the place of his kidneys since he was 12-years-old. His whole life, the 39-year-old says, revolves around dialysis.
“Whenever I try to plan anything, I also have to plan my dialysis,” says Burton says, who works as a freelance videographer and editor. “It’s a full-time job in itself.”
Many of those on dialysis are in line for a kidney transplant that would allow them to trade thrice-weekly dialysis and strict dietary limits for a lifetime of immunosuppressants. Burton’s previous transplant means that his body will likely reject another donated kidney unless it matches perfectly—something he’s not counting on. It’s why he’s enthusiastic about the development of artificial kidneys, small wearable or implantable devices that would do the job of a healthy kidney while giving users like Burton more flexibility for traveling, working, and more.
Still, the devices aren’t ready for testing in humans—yet. But recent advancements in engineering mean that the first preclinical trials for an artificial kidney could happen as soon as 18 months from now, according to Jonathan Himmelfarb, a nephrologist at the University of Washington.
“It would liberate people with kidney failure,” Himmelfarb says.
An engineering marvel
Compared to the heart or the brain, the kidney doesn’t get as much respect from the medical profession, but its job is far more complex. “It does hundreds of different things,” says UCLA’s Ira Kurtz.
Kurtz would know. He’s worked as a nephrologist for 37 years, devoting his career to helping those with kidney disease. While his colleagues in cardiology and endocrinology have seen major advances in the development of artificial hearts and insulin pumps, little has changed for patients on hemodialysis. The machines remain bulky and require large volumes of a liquid called dialysate to remove toxins from a patient’s blood, along with gallons of purified water. A kidney transplant is the next best thing to someone’s own, functioning organ, but with over 600,000 Americans on dialysis and only about 100,000 kidney transplants each year, most of those in kidney failure are stuck on dialysis.
Part of the lack of progress in artificial kidney design is the sheer complexity of the kidney’s job. Each of the 45 different cell types in the kidney do something different.
Part of the lack of progress in artificial kidney design is the sheer complexity of the kidney’s job. To build an artificial heart, Kurtz says, you basically need to engineer a pump. An artificial pancreas needs to balance blood sugar levels with insulin secretion. While neither of these tasks is simple, they are fairly straightforward. The kidney, on the other hand, does more than get rid of waste products like urea and other toxins. Each of the 45 different cell types in the kidney do something different, helping to regulate electrolytes like sodium, potassium, and phosphorous; maintaining blood pressure and water balance; guiding the body’s hormonal and inflammatory responses; and aiding in the formation of red blood cells.

There's been little progress for patients during Ira Kurtz's 37 years as a nephrologist. Artificial kidneys would change that.
UCLA
Dialysis primarily filters waste, and does so well enough to keep someone alive, but it isn’t a true artificial kidney because it doesn’t perform the kidney’s other jobs, according to Kurtz, such as sensing levels of toxins, wastes, and electrolytes in the blood. Due to the size and water requirements of existing dialysis machines, the equipment isn’t portable. Physicians write a prescription for a certain duration of dialysis and assess how well it’s working with semi-regular blood tests. The process of dialysis itself, however, is conducted blind. Doctors can’t tell how much dialysis a patient needs based on kidney values at the time of treatment, says Meera Harhay, a nephrologist at Drexel University in Philadelphia.
But it’s the impact of dialysis on their day-to-day lives that creates the most problems for patients. Only one-quarter of those on dialysis are able to remain employed (compared to 85% of similar-aged adults), and many report a low quality of life. Having more flexibility in life would make a major different to her patients, Harhay says.
“Almost half their week is taken up by the burden of their treatment. It really eats away at their freedom and their ability to do things that add value to their life,” she says.
Art imitates life
The challenge for artificial kidney designers was how to compress the kidney’s natural functions into a portable, wearable, or implantable device that wouldn’t need constant access to gallons of purified and sterilized water. The other universal challenge they faced was ensuring that any part of the artificial kidney that would come in contact with blood was kept germ-free to prevent infection.
As part of last year’s KidneyX Prize, a partnership between the U.S. Department of Health and Human Services and the American Society of Nephrology, inventors were challenged to create prototypes for artificial kidneys. Himmelfarb’s team at the University of Washington’s Center for Dialysis Innovation won the prize by focusing on miniaturizing existing technologies to create a portable dialysis machine. The backpack sized AKTIV device (Ambulatory Kidney to Increase Vitality) will recycle dialysate in a closed loop system that removes urea from blood and uses light-based chemical reactions to convert the urea to nitrogen and carbon dioxide, which allows the dialysate to be recirculated.
Himmelfarb says that the AKTIV can be used when at home, work, or traveling, which will give users more flexibility and freedom. “If you had a 30-pound device that you could put in the overhead bins when traveling, you could go visit your grandkids,” he says.
Kurtz’s team at UCLA partnered with the U.S. Kidney Research Corporation and Arkansas University to develop a dialysate-free desktop device (about the size of a small printer) as the first phase of a progression that will he hopes will lead to something small and implantable. Part of the reason for the artificial kidney’s size, Kurtz says, is the number of functions his team are cramming into it. Not only will it filter urea from blood, but it will also use electricity to help regulate electrolyte levels in a process called electrodeionization. Kurtz emphasizes that these additional functions are what makes his design a true artificial kidney instead of just a small dialysis machine.
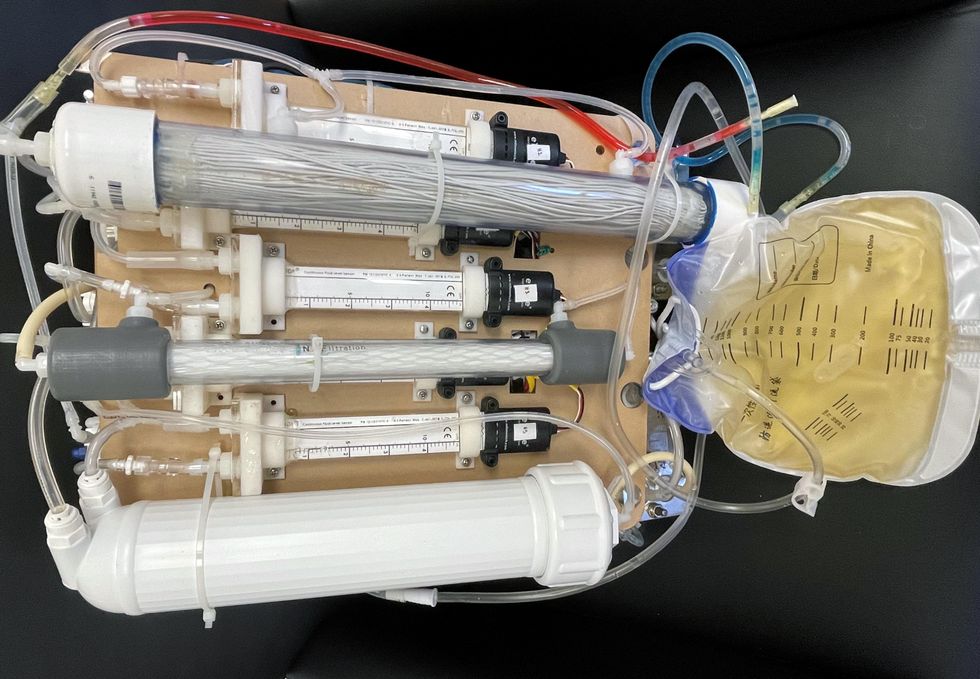
One version of an artificial kidney.
UCLA
“It doesn't have just a static function. It has a bank of sensors that measure chemicals in the blood and feeds that information back to the device,” Kurtz says.
Other startups are getting in on the game. Nephria Bio, a spinout from the South Korean-based EOFlow, is working to develop a wearable dialysis device, akin to an insulin pump, that uses miniature cartridges with nanomaterial filters to clean blood (Harhay is a scientific advisor to Nephria). Ian Welsford, Nephria’s co-founder and CTO, says that the device’s design means that it can also be used to treat acute kidney injuries in resource-limited settings. These potentials have garnered interest and investment in artificial kidneys from the U.S. Department of Defense.
For his part, Burton is most interested in an implantable device, as that would give him the most freedom. Even having a regular outpatient procedure to change batteries or filters would be a minor inconvenience to him.
“Being plugged into a machine, that’s not mimicking life,” he says.
A blood test may catch colorectal cancer before it's too late
A scientist works on a blood test in the Ajay Goel Lab, one of many labs that are developing blood tests to screen for different types of cancer.
Soon it may be possible to find different types of cancer earlier than ever through a simple blood test.
Among the many blood tests in development, researchers announced in July that they have developed one that may screen for early-onset colorectal cancer. The new potential screening tool, detailed in a study in the journal Gastroenterology, represents a major step in noninvasively and inexpensively detecting nonhereditary colorectal cancer at an earlier and more treatable stage.
In recent years, this type of cancer has been on the upswing in adults under age 50 and in those without a family history. In 2021, the American Cancer Society's revised guidelines began recommending that colorectal cancer screenings with colonoscopy begin at age 45. But that still wouldn’t catch many early-onset cases among people in their 20s and 30s, says Ajay Goel, professor and chair of molecular diagnostics and experimental therapeutics at City of Hope, a Los Angeles-based nonprofit cancer research and treatment center that developed the new blood test.
“These people will mostly be missed because they will never be screened for it,” Goel says. Overall, colorectal cancer is the fourth most common malignancy, according to the U.S. Centers for Disease Control and Prevention.
Goel is far from the only one working on this. Dozens of companies are in the process of developing blood tests to screen for different types of malignancies.
Some estimates indicate that between one-fourth and one-third of all newly diagnosed colorectal cancers are early-onset. These patients generally present with more aggressive and advanced disease at diagnosis compared to late-onset colorectal cancer detected in people 50 years or older.
To develop his test, Goel examined publicly available datasets and figured out that changes in novel microRNAs, or miRNAs, which regulate the expression of genes, occurred in people with early-onset colorectal cancer. He confirmed these biomarkers by looking for them in the blood of 149 patients who had the early-onset form of the disease. In particular, Goel and his team of researchers were able to pick out four miRNAs that serve as a telltale sign of this cancer when they’re found in combination with each other.
The blood test is being validated by following another group of patients with early-onset colorectal cancer. “We have filed for intellectual property on this invention and are currently seeking biotech/pharma partners to license and commercialize this invention,” Goel says.
He’s far from the only one working on this. Dozens of companies are in the process of developing blood tests to screen for different types of malignancies, says Timothy Rebbeck, a professor of cancer prevention at the Harvard T.H. Chan School of Public Health and the Dana-Farber Cancer Institute. But, he adds, “It’s still very early, and the technology still needs a lot of work before it will revolutionize early detection.”
The accuracy of the early detection blood tests for cancer isn’t yet where researchers would like it to be. To use these tests widely in people without cancer, a very high degree of precision is needed, says David VanderWeele, interim director of the OncoSET Molecular Tumor Board at Northwestern University’s Lurie Cancer Center in Chicago.
Otherwise, “you’re going to cause a lot of anxiety unnecessarily if people have false-positive tests,” VanderWeele says. So far, “these tests are better at finding cancer when there’s a higher burden of cancer present,” although the goal is to detect cancer at the earliest stages. Even so, “we are making progress,” he adds.
While early detection is known to improve outcomes, most cancers are detected too late, often after they metastasize and people develop symptoms. Only five cancer types have recommended standard screenings, none of which involve blood tests—breast, cervical, colorectal, lung (smokers considered at risk) and prostate cancers, says Trish Rowland, vice president of corporate communications at GRAIL, a biotechnology company in Menlo Park, Calif., which developed a multi-cancer early detection blood test.
These recommended screenings check for individual cancers rather than looking for any form of cancer someone may have. The devil lies in the fact that cancers without widespread screening recommendations represent the vast majority of cancer diagnoses and most cancer deaths.
GRAIL’s Galleri multi-cancer early detection test is designed to find more cancers at earlier stages by analyzing DNA shed into the bloodstream by cells—with as few false positives as possible, she says. The test is currently available by prescription only for those with an elevated risk of cancer. Consumers can request it from their healthcare or telemedicine provider. “Galleri can detect a shared cancer signal across more than 50 types of cancers through a simple blood draw,” Rowland says, adding that it can be integrated into annual health checks and routine blood work.
Cancer patients—even those with early and curable disease—often have tumor cells circulating in their blood. “These tumor cells act as a biomarker and can be used for cancer detection and diagnosis,” says Andrew Wang, a radiation oncologist and professor at the University of Texas Southwestern Medical Center in Dallas. “Our research goal is to be able to detect these tumor cells to help with cancer management.” Collaborating with Seungpyo Hong, the Milton J. Henrichs Chair and Professor at the University of Wisconsin-Madison School of Pharmacy, “we have developed a highly sensitive assay to capture these circulating tumor cells.”
Even if the quality of a blood test is superior, finding cancer early doesn’t always mean it’s absolutely best to treat it. For example, prostate cancer treatment’s potential side effects—the inability to control urine or have sex—may be worse than living with a slow-growing tumor that is unlikely to be fatal. “[The test] needs to tell me, am I going to die of that cancer? And, if I intervene, will I live longer?” says John Marshall, chief of hematology and oncology at Medstar Georgetown University Hospital in Washington, D.C.

Ajay Goel Lab
A blood test developed at the University of Texas MD Anderson Cancer Center in Houston helps predict who may benefit from lung cancer screening when it is combined with a risk model based on an individual’s smoking history, according to a study published in January in the Journal of Clinical Oncology. The personalized lung cancer risk assessment was more sensitive and specific than the 2021 and 2013 U.S. Preventive Services Task Force criteria.
The study involved participants from the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial with a minimum of a 10 pack-year smoking history, meaning they smoked 20 cigarettes per day for ten years. If implemented, the blood test plus model would have found 9.2 percent more lung cancer cases for screening and decreased referral to screening among non-cases by 13.7 percent compared to the 2021 task force criteria, according to Oncology Times.
The conventional type of screening for lung cancer is an annual low-dose CT scan, but only a small percentage of people who are eligible will actually get these scans, says Sam Hanash, professor of clinical cancer prevention and director of MD Anderson’s Center for Global Cancer Early Detection. Such screening is not readily available in most countries.
In methodically searching for blood-based biomarkers for lung cancer screening, MD Anderson researchers developed a simple test consisting of four proteins. These proteins circulating in the blood were at high levels in individuals who had lung cancer or later developed it, Hanash says.
“The interest in blood tests for cancer early detection has skyrocketed in the past few years,” he notes, “due in part to advances in technology and a better understanding of cancer causation, cancer drivers and molecular changes that occur with cancer development.”
However, at the present time, none of the blood tests being considered eliminate the need for screening of eligible subjects using established methods, such as colonoscopy for colorectal cancer. Yet, Hanash says, “they have the potential to complement these modalities.”
Study Shows “Living Drug” Can Provide a Lasting Cure for Cancer
A recent study by researchers at the University of Pennsylvania examined how CAR-T therapy helped Doug Olson beat a cancer death sentence for over a decade - and how it could work for more people.
Doug Olson was 49 when he was diagnosed with chronic lymphocytic leukemia, a blood cancer that strikes 21,000 Americans annually. Although the disease kills most patients within a decade, Olson’s case progressed more slowly, and courses of mild chemotherapy kept him healthy for 13 years. Then, when he was 62, the medication stopped working. The cancer had mutated, his doctor explained, becoming resistant to standard remedies. Harsher forms of chemo might buy him a few months, but their side effects would be debilitating. It was time to consider the treatment of last resort: a bone-marrow transplant.
Olson, a scientist who developed blood-testing instruments, knew the odds. There was only a 50 percent chance that a transplant would cure him. There was a 20 percent chance that the agonizing procedure—which involves destroying the patient’s marrow with chemo and radiation, then infusing his blood with donated stem cells—would kill him. If he survived, he would face the danger of graft-versus-host disease, in which the donor’s cells attack the recipient’s tissues. To prevent it, he would have to take immunosuppressant drugs, increasing the risk of infections. He could end up with pneumonia if one of his three grandchildren caught a sniffle. “I was being pushed into a corner,” Olson recalls, “with very little room to move.”
Soon afterward, however, his doctor revealed a possible escape route. He and some colleagues at the University of Pennsylvania’s Abramson Cancer Center were starting a clinical trial, he said, and Olson—still mostly symptom-free—might be a good candidate. The experimental treatment, known as CAR-T therapy, would use genetic engineering to turn his T lymphocytes (immune cells that guard against viruses and other pathogens) into a weapon against cancer.
In September 2010, technicians took some of Olson’s T cells to a laboratory, where they were programmed with new molecular marching orders and coaxed to multiply into an army of millions. When they were ready, a nurse inserted a catheter into his neck. At the turn of a valve, his soldiers returned home, ready to do battle.
“I felt like I’d won the lottery,” Olson says. But he was only the second person in the world to receive this “living drug,” as the University of Pennsylvania investigators called it. No one knew how long his remission would last.
Three weeks later, Olson was slammed with a 102-degree fever, nausea, and chills. The treatment had triggered two dangerous complications: cytokine release syndrome, in which immune chemicals inflame the patient’s tissues, and tumor lysis syndrome, in which toxins from dying cancer cells overwhelm the kidneys. But the crisis passed quickly, and the CAR-T cells fought on. A month after the infusion, the doctor delivered astounding news: “We can’t find any cancer in your body.”
“I felt like I’d won the lottery,” Olson says. But he was only the second person in the world to receive this “living drug,” as the University of Pennsylvania investigators called it. No one knew how long his remission would last.
An Unexpected Cure
In February 2022, the same cancer researchers reported a remarkable milestone: the trial’s first two patients had survived for more than a decade. Although Olson’s predecessor—a retired corrections officer named Bill Ludwig—died of COVID-19 complications in early 2021, both men had remained cancer-free. And the modified immune cells continued to patrol their territory, ready to kill suspected tumor cells the moment they arose.
“We can now conclude that CAR-T cells can actually cure patients with leukemia,” University of Pennsylvania immunologist Carl June, who spearheaded the development of the technique, told reporters. “We thought the cells would be gone in a month or two. The fact that they’ve survived 10 years is a major surprise.”
Even before the announcement, it was clear that CAR-T therapy could win a lasting reprieve for many patients with cancers that were once a death sentence. Since the Food and Drug Administration approved June’s version (marketed as Kymriah) in 2017, the agency has greenlighted five more such treatments for various types of leukemia, lymphoma, and myeloma. “Every single day, I take care of patients who would previously have been told they had no options,” says Rayne Rouce, a pediatric hematologist/oncologist at Texas Children’s Cancer Center. “Now we not only have a treatment option for those patients, but one that could potentially be the last therapy for their cancer that they’ll ever have to receive.”

Immunologist Carl June, middle, spearheaded development of the CAR-T therapy that gave patients Bill Ludwig, left, and Doug Olson, right, a lengthy reprieve on their terminal cancer diagnoses.
Penn Medicine
Yet the CAR-T approach doesn’t help everyone. So far, it has only shown success for blood cancers—and for those, the overall remission rate is 30 to 40 percent. “When it works, it works extraordinarily well,” says Olson’s former doctor, David Porter, director of Penn’s blood and bone marrow transplant program. “It’s important to know why it works, but it’s equally important to know why it doesn’t—and how we can fix that.”
The team’s study, published in the journal Nature, offers a wealth of data on what worked for these two patients. It may also hold clues for how to make the therapy effective for more people.
Building a Better T Cell
Carl June didn’t set out to cure cancer, but his serendipitous career path—and a personal tragedy—helped him achieve insights that had eluded other researchers. In 1971, hoping to avoid combat in Vietnam, he applied to the U.S. Naval Academy in Annapolis, Maryland. June showed a knack for biology, so the Navy sent him on to Baylor College of Medicine. He fell in love with immunology during a fellowship researching malaria vaccines in Switzerland. Later, the Navy deployed him to the Fred Hutchinson Cancer Research Center in Seattle to study bone marrow transplantation.
There, June became part of the first research team to learn how to culture T cells efficiently in a lab. After moving on to the National Naval Medical Center in the ’80s, he used that knowledge to combat the newly emerging AIDS epidemic. HIV, the virus that causes the disease, invades T cells and eventually destroys them. June and his post-doc Bruce Levine developed a method to restore patients’ depleted cell populations, using tiny magnetic beads to deliver growth-stimulating proteins. Infused into the body, the new T cells effectively boosted immune function.
In 1999, after leaving the Navy, June joined the University of Pennsylvania. His wife, who’d been diagnosed with ovarian cancer, died two years later, leaving three young children. “I had not known what it was like to be on the other side of the bed,” he recalls. Watching her suffer through grueling but futile chemotherapy, followed by an unsuccessful bone-marrow transplant, he resolved to focus on finding better cancer treatments. He started with leukemia—a family of diseases in which mutant white blood cells proliferate in the marrow.
Cancer is highly skilled at slipping through the immune system’s defenses. T cells, for example, detect pathogens by latching onto them with receptors designed to recognize foreign proteins. Leukemia cells evade detection, in part, by masquerading as normal white blood cells—that is, as part of the immune system itself.
June planned to use a viral vector no one had tried before: HIV.
To June, chimeric antigen receptor (CAR) T cells looked like a promising tool for unmasking and destroying the impostors. Developed in the early ’90s, these cells could be programmed to identify a target protein, and to kill any pathogen that displayed it. To do the programming, you spliced together snippets of DNA and inserted them into a disabled virus. Next, you removed some of the patient’s T cells and infected them with the virus, which genetically hijacked its new hosts—instructing them to find and slay the patient’s particular type of cancer cells. When the T cells multiplied, their descendants carried the new genetic code. You then infused those modified cells into the patient, where they went to war against their designated enemy.
Or that’s what happened in theory. Many scientists had tried to develop therapies using CAR-T cells, but none had succeeded. Although the technique worked in lab animals, the cells either died out or lost their potency in humans.
But June had the advantage of his years nurturing T cells for AIDS patients, as well as the technology he’d developed with Levine (who’d followed him to Penn with other team members). He also planned to use a viral vector no one had tried before: HIV, which had evolved to thrive in human T cells and could be altered to avoid causing disease. By the summer of 2010, he was ready to test CAR-T therapy against chronic lymphocytic leukemia (CLL), the most common form of the disease in adults.
Three patients signed up for the trial, including Doug Olson and Bill Ludwig. A portion of each man’s T cells were reprogrammed to detect a protein found only on B lymphocytes, the type of white blood cells affected by CLL. Their genetic instructions ordered them to destroy any cell carrying the protein, known as CD19, and to multiply whenever they encountered one. This meant the patients would forfeit all their B cells, not just cancerous ones—but regular injections of gamma globulins (a cocktail of antibodies) would make up for the loss.
After being infused with the CAR-T cells, all three men suffered high fevers and potentially life-threatening inflammation, but all pulled through without lasting damage. The third patient experienced a partial remission and survived for eight months. Olson and Ludwig were cured.
Learning What Works
Since those first infusions, researchers have developed reliable ways to prevent or treat the side effects of CAR-T therapy, greatly reducing its risks. They’ve also been experimenting with combination therapies—pairing CAR-T with chemo, cancer vaccines, and immunotherapy drugs called checkpoint inhibitors—to improve its success rate. But CAR-T cells are still ineffective for at least 60 percent of blood cancer patients. And they remain in the experimental stage for solid tumors (including pancreatic cancer, mesothelioma, and glioblastoma), whose greater complexity make them harder to attack.
The new Nature study offers clues that could fuel further advances. The Penn team “profiled these cells at a level where we can almost say, ‘These are the characteristics that a T cell would need to survive 10 years,’” says Rouce, the physician at Texas Children’s Cancer Center.
One surprising finding involves how CAR-T cells change in the body over time. At first, those that Olson and Ludwig received showed the hallmarks of “killer” T-cells (also known as CD8 cells)—highly active lymphocytes bent on exterminating every tumor cell in sight. After several months, however, the population shifted toward “helper” T-cells (or CD4s), which aid in forming long-term immune memory but are normally incapable of direct aggression. Over the years, the numbers swung back and forth, until only helper cells remained. Those cells showed markers suggesting they were too exhausted to function—but in the lab, they were able not only to recognize but to destroy cancer cells.
June and his team suspect that those tired-looking helper cells had enough oomph to kill off any B cells Olson and Ludwig made, keeping the pair’s cancers permanently at bay. If so, that could prompt new approaches to selecting cells for CAR-T therapy. Maybe starting with a mix of cell types—not only CD8s, but CD4s and other varieties—would work better than using CD8s alone. Or perhaps inducing changes in cell populations at different times would help.
Another potential avenue for improvement is starting with healthier cells. Evidence from this and other trials hints that patients whose T cells are more robust to begin with respond better when their cells are used in CAR-T therapy. The Penn team recently completed a clinical trial in which CLL patients were treated with ibrutinib—a drug that enhances T-cell function—before their CAR-T cells were manufactured. The response rate, says David Porter, was “very high,” with most patients remaining cancer-free a year after being infused with the souped-up cells.
Such approaches, he adds, are essential to achieving the next phase in CAR-T therapy: “Getting it to work not just in more people, but in everybody.”

Doug Olson enjoys nature - and having a future.
Penn Medicine
To grasp what that could mean, it helps to talk with Doug Olson, who’s now 75. In the years since his infusion, he has watched his four children forge careers, and his grandkids reach their teens. He has built a business and enjoyed the rewards of semi-retirement. He’s done volunteer and advocacy work for cancer patients, run half-marathons, sailed the Caribbean, and ridden his bike along the sun-dappled roads of Silicon Valley, his current home.
And in his spare moments, he has just sat there feeling grateful. “You don’t really appreciate the effect of having a lethal disease until it’s not there anymore,” he says. “The world looks different when you have a future.”
This article was first published on Leaps.org on March 24, 2022.

