A vaccine for Lyme disease could be coming. But will patients accept it?

Hiking is one of Marci Flory's favorite activities, but her chronic Lyme disease sometimes renders her unable to walk. Biotech companies Pfizer and Valneva are testing a vaccine for Lyme disease in a Phase III clinical trial.
For more than two decades, Marci Flory, a 40-year-old emergency room nurse from Lawrence, Kan., has battled the recurring symptoms of chronic Lyme disease, an illness which she believes began after being bitten by a tick during her teenage years.
Over the years, Flory has been plagued by an array of mysterious ailments, ranging from fatigue to crippling pain in her eyes, joints and neck, and even postural tachycardia syndrome or PoTS, an abnormal increase in heart rate after sitting up or standing. Ten years ago, she began to experience the onset of neurological symptoms which ranged from brain fog to sudden headaches, and strange episodes of leg weakness which would leave her unable to walk.
“Initially doctors thought I had ALS, or less likely, multiple sclerosis,” she says. “But after repeated MRI scans for a year, they concluded I had a rare neurological condition called acute transverse myelitis.”
But Flory was not convinced. After ordering a variety of private blood tests, she discovered she was infected with a range of bacteria in the genus Borrelia that live in the guts of ticks, the infectious agents responsible for Lyme disease.
“It made sense,” she says. “Looking back, I was bitten in high school and misdiagnosed with mononucleosis. This was probably the start, and my immune system kept it under wraps for a while. The Lyme bacteria can burrow into every tissue in the body, go into cyst form and become dormant before reactivating.”
The reason why cases of Lyme disease are increasing is down to changing weather patterns, triggered by climate change, meaning that ticks are now found across a much wider geographic range than ever before.
When these species of bacteria are transmitted to humans, they can attack the nervous system, joints and even internal organs which can lead to serious health complications such as arthritis, meningitis and even heart failure. While Lyme disease can sometimes be successfully treated with antibiotics if spotted early on, not everyone responds to these drugs, and for patients who have developed chronic symptoms, there is no known cure. Flory says she knows of fellow Lyme disease patients who have spent hundreds of thousands of dollars seeking treatments.
Concerningly, statistics show that Lyme and other tick-borne diseases are on the rise. Recently released estimates based on health insurance records suggest that at least 476,000 Americans are diagnosed with Lyme disease every year, and many experts believe the true figure is far higher.
The reason why the numbers are growing is down to changing weather patterns, triggered by climate change, meaning that ticks are now found across a much wider geographic range than ever before. Health insurance data shows that cases of Lyme disease have increased fourfold in rural parts of the U.S. over the last 15 years, and 65 percent in urban regions.
As a result, many scientists who have studied Lyme disease feel that it is paramount to bring some form of protective vaccine to market which can be offered to people living in the most at-risk areas.
“Even the increased awareness for Lyme disease has not stopped the cases,” says Eva Sapi, professor of cellular and molecular biology at the University of New Haven. “Some of these patients are looking for answers for years, running from one doctor to another, so that is obviously a very big cost for our society at so many levels.”
Emerging vaccines – and backlash
But with the rising case numbers, interest has grown among the pharmaceutical industry and research communities. Vienna-based biotech Valneva have partnered with Pfizer to take their vaccine – a seasonal jab which offers protection against the six most common strains of Lyme disease in the northern hemisphere – into a Phase III clinical trial which began in August. Involving 6,000 participants in a number of U.S. states and northern Europe where Lyme disease is endemic, it could lead to a licensed vaccine by 2025, if it proves successful.
“For many years Lyme was considered a small market vaccine,” explains Monica E. Embers, assistant professor of parasitology at Tulane University in New Orleans. “Now we know that this is a much bigger problem, Pfizer has stepped up to invest in preventing this disease and other pharmaceutical companies may as well.”
Despite innovations, patient communities and their representatives remain ambivalent about the idea of a vaccine. Some of this skepticism dates back to the failed LYMErix vaccine which was developed in the late 1990s before being withdrawn from the market.
At the same time, scientists at Yale University are developing a messenger RNA vaccine which aims to train the immune system to respond to tick bites by exposing it to 19 proteins found in tick saliva. Whereas the Valneva vaccine targets the bacteria within ticks, the Yale vaccine attempts to provoke an instant and aggressive immune response at the site of the bite. This causes the tick to fall off and limits the potential for transmitting dangerous infections.
But despite these innovations, patient communities and their representatives remain ambivalent about the idea of a vaccine. Some of this skepticism dates back to the failed LYMErix vaccine which was developed in the late 1990s before being withdrawn from the market in 2002 after concerns were raised that it might induce autoimmune reactions in humans.
While this theory was ultimately disproved, the lingering stigma attached to LYMErix meant that most vaccine manufacturers chose to stay away from the disease for many years, something which Gregory Poland, head of the Mayo Clinic’s Vaccine Research Group in Minnesota, describes as a tragedy.
“Since 2002, we have not had a human Lyme vaccine in the U.S. despite the increasing number of cases,” says Poland. “Pretty much everyone in the field thinks they’re ten times higher than the official numbers, so you’re probably talking at least 400,000 each year. It’s an incredible burden but because of concerns about anti-vax protestors, until very recently, no manufacturer has wanted to touch this.”
Such was the backlash surrounding the failed LYMErix program that scientists have even explored the most creative of workarounds for protecting people in tick-populated regions, without needing to actually vaccinate them. One research program at the University of Tennessee came up with the idea of leaving food pellets containing a vaccine in woodland areas with the idea that rodents would eat the pellets, and the vaccine would then kill Borrelia bacteria within any ticks which subsequently fed on the animals.
Even the Pfizer-Valneva vaccine has been cautiously designed to try and allay any lingering concerns, two decades after LYMErix. “The concept is the same as the original LYMErix vaccine, but it has been made safer by removing regions that had the potential to induce autoimmunity,” says Embers. “There will always be individuals who oppose vaccines, Lyme or otherwise, but it will be a tremendous boost to public health to have the option.”
Vaccine alternatives
Researchers are also considering alternative immunization approaches in case sufficiently large numbers of people choose to reject any Lyme vaccine which gets approved. Researchers at UMass Chan Medical School have developed an artificially generated antibody, administered via an annual injection, which is capable of killing Borrelia bacteria in the guts of ticks before they can get into the human host.
So far animal studies have shown it to be 100 percent effective, while the scientists have completed a Phase I trial in which they tested it for safety on 48 volunteers in Nebraska. Because this approach provides the antibody directly, rather than triggering the human immune system to produce the antibody like a vaccine would, Embers predicts that it could be a viable alternative for the vaccine hesitant as well as providing an option for immunocompromised individuals who cannot produce enough of their own antibodies.
At the same time, many patient groups still raise concerns over the fact that numerous diagnostic tests for Lyme disease have been reported to have a poor accuracy. Without this, they argue that it is difficult to prove whether vaccines or any other form of immunization actually work. “If the disease is not understood enough to create a more accurate test and a universally accepted treatment protocol, particularly for those who weren’t treated promptly, how can we be sure about the efficacy of a vaccine?” says Natasha Metcalf, co-founder of the organization Lyme Disease UK.
Flory points out that there are so many different types of Borrelia bacteria which cause Lyme disease, that the immunizations being developed may only stop a proportion of cases. In addition, she says that chronic Lyme patients often report a whole myriad of co-infections which remain poorly understood and are likely to also be involved in the disease process.
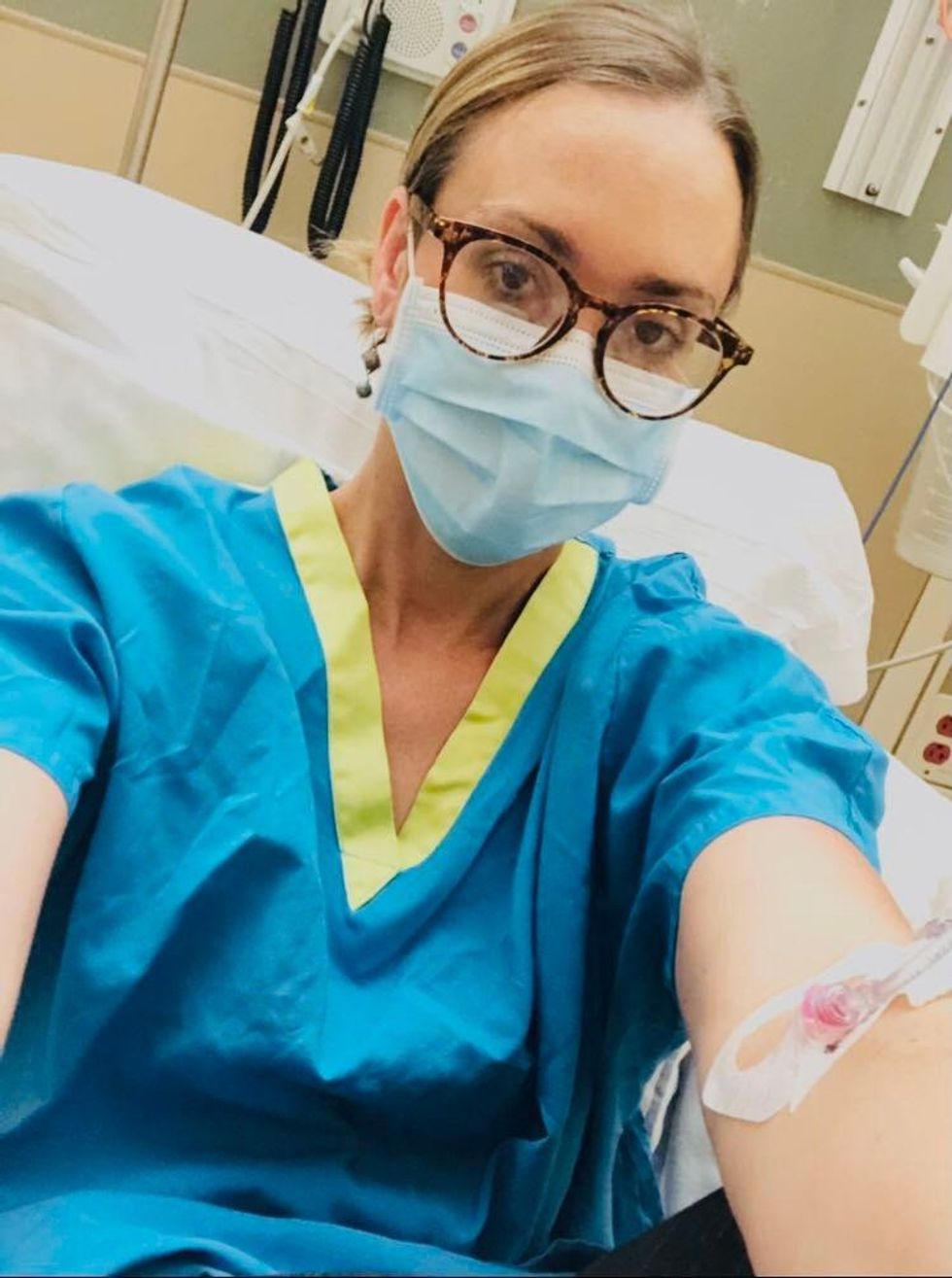
Marci Flory undergoes an infusion in an attempt to treat her Lyme disease symptoms.
Marci Flory
“I would love to see an effective Lyme vaccine but I have my reservations,” she says. “I am infected with four types of Borrelia bacteria, plus many co-infections – Babesia, Bartonella, Erlichiosis, Rickettsia, and Mycoplasma – all from a single Douglas County Kansas tick bite. Lyme never travels alone and the vaccine won’t protect against all the many strains of Borrelia and co-infections.”
Valneva CEO Thomas Lingelbach admits that the Pfizer-Valneva vaccine is not perfect, but predicts that it will still have significant impact if approved.
“We expect the vaccine to have 75 percent plus efficacy,” he says. “There is this legacy around the old Lyme vaccines, but the world is very, very different today. The number of clinical manifestations known to be caused by infection with Lyme Borreliosis has significantly increased, and the understanding around severity has certainly increased.”
Embers agrees that while it will still be important for doctors to monitor for other tick-borne infections which are not necessarily covered by the vaccine, having any clinically approved jab would still represent a major step forward in the fight against the disease.
“I think that any vaccine must be properly vetted, and these companies are performing extensive clinical trials to do just that,” she says. “Lyme is the most common tick-borne disease in the U.S. so the public health impact could be significant. However, clinicians and the general public must remain aware of all of the other tick-borne diseases such as Babesia and Anaplasma, and continue to screen for those when a tick bite is suspected.”
Henrietta Lacks' Cells Enabled Medical Breakthroughs. Is It Time to Finally Retire Them?
Henrietta Lacks, (1920-1951) unknowingly had her cells cultured and used in medical research.
For Victoria Tokarz, a third-year PhD student at the University of Toronto, experimenting with cells is just part of a day's work. Tokarz, 26, is studying to be a cell biologist and spends her time inside the lab manipulating muscle cells sourced from rodents to try to figure out how they respond to insulin. She hopes this research could someday lead to a breakthrough in our understanding of diabetes.
"People like to use HeLa cells because they're easy to use."
But in all her research, there is one cell culture that Tokarz refuses to touch. The culture is called HeLa, short for Henrietta Lacks, named after the 31-year-old tobacco farmer the cells were stolen from during a tumor biopsy she underwent in 1951.
"In my opinion, there's no question or experiment I can think of that validates stealing from and profiting off of a black woman's body," Tokarz says. "We're not talking about a reagent we created in a lab, a mixture of some chemicals. We're talking about a human being who suffered indescribably so we could profit off of her misfortune."
Lacks' suffering is something that, until recently, was not widely known. Born to a poor family in Roanoke, VA, Lacks was sent to live with her grandfather on the family tobacco farm at age four, shortly after the death of her mother. She gave birth to her first child at just fourteen, and two years later had another child with profound developmental disabilities. Lacks married her first cousin, David, in 1941 and the family moved to Maryland where they had three additional children.
But the real misfortune came in 1951, when Lacks told her cousins that she felt a hard "knot" in her womb. When Lacks went to Johns Hopkins hospital to have the knot examined, doctors discovered that the hard lump Henrietta felt was a rapidly-growing cervical tumor.
Before the doctors treated the tumor – inserting radium tubes into her vagina, in the hopes they could kill the cancer, Lacks' doctors clipped two tissue samples from her cervix, without Lacks' knowledge or consent. While it's considered widely unethical today, taking tissue samples from patients was commonplace at the time. The samples were sent to a cancer researcher at Johns Hopkins and Lacks continued treatment unsuccessfully until she died a few months later of metastatic cancer.
Lacks' story was not over, however: When her tissue sample arrived at the lab of George Otto Gey, the Johns Hopkins cancer researcher, he noticed that the cancerous cells grew at a shocking pace. Unlike other cell cultures that would die within a day or two of arriving at the lab, Lacks' cells kept multiplying. They doubled every 24 hours, and to this day, have never stopped.
Scientists would later find out that this growth was due to an infection of Human Papilloma Virus, or HPV, which is known for causing aggressive cancers. Lacks' cells became the world's first-ever "immortalized" human cell line, meaning that as long as certain environmental conditions are met, the cells can replicate indefinitely. Although scientists have cultivated other immortalized cell lines since then, HeLa cells remain a favorite among scientists due to their resilience, Tokarz says.
"People like to use HeLa cells because they're easy to use," Tokarz says. "They're easy to manipulate, because they're very hardy, and they allow for transection, which means expressing a protein in a cell that's not normally there. Other cells, like endothelial cells, don't handle those manipulations well."
Once the doctors at Johns Hopkins discovered that Lacks' cells could replicate indefinitely, they started shipping them to labs around the world to promote medical research. As they were the only immortalized cell line available at the time, researchers used them for thousands of experiments — some of which resulted in life-saving treatments. Jonas Salk's polio vaccine, for example, was manufactured using HeLa cells. HeLa cell research was also used to develop a vaccine for HPV, and for the development of in vitro fertilization and gene mapping. Between 1951 and 2018, HeLa cells have been cited in over 110,000 publications, according to a review from the National Institutes of Health.
But while some scientists like Tokarz are thankful for the advances brought about by HeLa cells, they still believe it's well past time to stop using them in research.
"Am I thankful we have a polio vaccine? Absolutely. Do I resent the way we came to have that vaccine? Absolutely," Tokarz says. "We could have still arrived at those same advances by treating her as the human being she is, not just a specimen."
Ethical considerations aside, HeLa is no longer the world's only available cell line – nor, Tokarz argues, are her cells the most suitable for every type of research. "The closer you can get to the physiology of the thing you're studying, the better," she says. "Now we have the ability to use primary cells, which are isolated from a person and put right into the culture dish, and those don't have the same mutations as cells that have been growing for 20 years. We didn't have the expertise to do that initially, but now we do."
Raphael Valdivia, a professor of molecular genetics and microbiology at Duke University School of Medicine, agrees that HeLa cells are no longer optimal for most research. "A lot of scientists are moving away from HeLa cells because they're so unstable," he says. "They mutate, they rearrange chromosomes to become adaptive, and different batches of cells evolve separately from each other. The HeLa cells in my lab are very different than the ones down the hall, and that means sometimes we can't replicate our results. We have to go back to an earlier batch of cells in the freezer and re-test."
Still, the idea of retiring the cells completely doesn't make sense, Valdivia says: "To some extent, you're beholden to previous research. You need to be able to confirm findings that happen in earlier studies, and to do that you need to use the same cell line that other researchers have used."
"Ethics is not black and white, and sometimes there's no such thing as a straightforward ethical or unethical choice."
"The way in which the cells were taken – without patient consent – is completely inappropriate," says Yann Joly, associate professor at the Faculty of Medicine in Toronto and Research Director at the Centre of Genomics and Policy. "The question now becomes, what can we do about it now? What are our options?"
While scientists are not able to erase what was done to Henrietta Lacks, Joly argues that retiring her cells is also non-consensual, assuming – maybe incorrectly – what Henrietta would have wanted, without her input. Additionally, Joly points out that other immortalized human cell lines are fraught with what some people consider to be ethical concerns as well, such as the human embryonic kidney cell line, commonly referred to as HEK-293, that was derived from an aborted female fetus. "Just because you're using another kind of cell doesn't mean it's devoid of ethical issue," he says.
Seemingly, the one thing scientists can agree on is that Henrietta Lacks was mistreated by the medical community. But even so, retiring her cells from medical research is not an obvious solution. Scientists are now using HeLa cells to better understand how the novel coronavirus affects humans, and this knowledge will inform how researchers develop a COVID-19 vaccine.
"Ethics is not black and white, and sometimes there's no such thing as a straightforward ethical or unethical choice," Joly says. "If [ethics] were that easy, nobody would need to teach it."
An intergalactic explorer on a hypothetical mission to Mars.
Social isolation. Strange pathogens outside. Strategic resource planning. Our Earthbound pandemic-driven social distancing could be mistaken for adapting to another, foreign planet. After all, we're donning all our protective apparel to go on an airplane or to the grocery store, nevertheless to just open our front door. Perhaps this is training for the world galactic visionaries Elon Musk, Jeff Bezos, and Richard Branson see in our future.
"There are parallels to the individual psychological experience, but from an operational standpoint, it is too different."
Ready to go live on Mars or something? Not so fast, experts say. The experience of shelter in place isn't parallel to being a space settler, or even an astronaut.
"Certain aspects are similar, but still, honestly, there are too many differences to say it preps us," says Angelo Vermeulen, co-founder of the art-science collective SEADS (Space Ecologies Art and Design) Network. In 2013, he served as a NASA crew commander for a four-month Mars-on-Earth mission, isolated in a geometric biodome with five others. "There are parallels to the individual psychological experience, but from an operational standpoint, it is too different. You don't need a spacesuit, aren't threatened by a thin atmosphere or worried about being overpowered by radiation."
Outside threats aside, we have a bigger experience gap: Most of us didn't see this pandemic coming and weren't trained to survive the current new normal. NASA astronauts get at least two years of basic training. We received none. Intergalactic explorers understand gravity, air pressure, and other important criteria based on decades of space knowledge. Alternatively, new novel coronavirus data is coming in real time, changing the threats, precautions, and needs dramatically. Things feel a little different when you're winging it.
Lastly, with respect to Apollo 13, space travelers have a timeline for when their experience will be over. There are mishaps, challenges and adjustments, but every well-supported journeyperson leaves Earth with an agenda (and a team back home to help keep them on track).
The pandemic, on the other hand, has no definitive end. It is unclear when a reliable vaccine will be readily available. It is also not known how long we should shelter-in-place, as pulling the trigger too early could bring another wave of illness. We are missing definitive milestones, which, Vermeulen says, would make our isolation experience easier to navigate. "When you're on a mission, the end date is always on the horizon. You can celebrate the midpoint and check off major milestones, which helps."
Also, unlike a kid pretending to be in a rocket, most of us didn't dream of one day being socially isolated for an indeterminate amount of time. "If you're ambitious and working in the field, then it is your goal in life to experience [space and the related isolation]," he says. "With the pandemic, though, nobody chose to do this."
[Editor's Note: This article was originally published on June 8th, 2020 as part of a standalone magazine called GOOD10: The Pandemic Issue. Produced as a partnership among LeapsMag, The Aspen Institute, and GOOD, the magazine is available for free online.]

