A vaccine for Lyme disease could be coming. But will patients accept it?

Hiking is one of Marci Flory's favorite activities, but her chronic Lyme disease sometimes renders her unable to walk. Biotech companies Pfizer and Valneva are testing a vaccine for Lyme disease in a Phase III clinical trial.
For more than two decades, Marci Flory, a 40-year-old emergency room nurse from Lawrence, Kan., has battled the recurring symptoms of chronic Lyme disease, an illness which she believes began after being bitten by a tick during her teenage years.
Over the years, Flory has been plagued by an array of mysterious ailments, ranging from fatigue to crippling pain in her eyes, joints and neck, and even postural tachycardia syndrome or PoTS, an abnormal increase in heart rate after sitting up or standing. Ten years ago, she began to experience the onset of neurological symptoms which ranged from brain fog to sudden headaches, and strange episodes of leg weakness which would leave her unable to walk.
“Initially doctors thought I had ALS, or less likely, multiple sclerosis,” she says. “But after repeated MRI scans for a year, they concluded I had a rare neurological condition called acute transverse myelitis.”
But Flory was not convinced. After ordering a variety of private blood tests, she discovered she was infected with a range of bacteria in the genus Borrelia that live in the guts of ticks, the infectious agents responsible for Lyme disease.
“It made sense,” she says. “Looking back, I was bitten in high school and misdiagnosed with mononucleosis. This was probably the start, and my immune system kept it under wraps for a while. The Lyme bacteria can burrow into every tissue in the body, go into cyst form and become dormant before reactivating.”
The reason why cases of Lyme disease are increasing is down to changing weather patterns, triggered by climate change, meaning that ticks are now found across a much wider geographic range than ever before.
When these species of bacteria are transmitted to humans, they can attack the nervous system, joints and even internal organs which can lead to serious health complications such as arthritis, meningitis and even heart failure. While Lyme disease can sometimes be successfully treated with antibiotics if spotted early on, not everyone responds to these drugs, and for patients who have developed chronic symptoms, there is no known cure. Flory says she knows of fellow Lyme disease patients who have spent hundreds of thousands of dollars seeking treatments.
Concerningly, statistics show that Lyme and other tick-borne diseases are on the rise. Recently released estimates based on health insurance records suggest that at least 476,000 Americans are diagnosed with Lyme disease every year, and many experts believe the true figure is far higher.
The reason why the numbers are growing is down to changing weather patterns, triggered by climate change, meaning that ticks are now found across a much wider geographic range than ever before. Health insurance data shows that cases of Lyme disease have increased fourfold in rural parts of the U.S. over the last 15 years, and 65 percent in urban regions.
As a result, many scientists who have studied Lyme disease feel that it is paramount to bring some form of protective vaccine to market which can be offered to people living in the most at-risk areas.
“Even the increased awareness for Lyme disease has not stopped the cases,” says Eva Sapi, professor of cellular and molecular biology at the University of New Haven. “Some of these patients are looking for answers for years, running from one doctor to another, so that is obviously a very big cost for our society at so many levels.”
Emerging vaccines – and backlash
But with the rising case numbers, interest has grown among the pharmaceutical industry and research communities. Vienna-based biotech Valneva have partnered with Pfizer to take their vaccine – a seasonal jab which offers protection against the six most common strains of Lyme disease in the northern hemisphere – into a Phase III clinical trial which began in August. Involving 6,000 participants in a number of U.S. states and northern Europe where Lyme disease is endemic, it could lead to a licensed vaccine by 2025, if it proves successful.
“For many years Lyme was considered a small market vaccine,” explains Monica E. Embers, assistant professor of parasitology at Tulane University in New Orleans. “Now we know that this is a much bigger problem, Pfizer has stepped up to invest in preventing this disease and other pharmaceutical companies may as well.”
Despite innovations, patient communities and their representatives remain ambivalent about the idea of a vaccine. Some of this skepticism dates back to the failed LYMErix vaccine which was developed in the late 1990s before being withdrawn from the market.
At the same time, scientists at Yale University are developing a messenger RNA vaccine which aims to train the immune system to respond to tick bites by exposing it to 19 proteins found in tick saliva. Whereas the Valneva vaccine targets the bacteria within ticks, the Yale vaccine attempts to provoke an instant and aggressive immune response at the site of the bite. This causes the tick to fall off and limits the potential for transmitting dangerous infections.
But despite these innovations, patient communities and their representatives remain ambivalent about the idea of a vaccine. Some of this skepticism dates back to the failed LYMErix vaccine which was developed in the late 1990s before being withdrawn from the market in 2002 after concerns were raised that it might induce autoimmune reactions in humans.
While this theory was ultimately disproved, the lingering stigma attached to LYMErix meant that most vaccine manufacturers chose to stay away from the disease for many years, something which Gregory Poland, head of the Mayo Clinic’s Vaccine Research Group in Minnesota, describes as a tragedy.
“Since 2002, we have not had a human Lyme vaccine in the U.S. despite the increasing number of cases,” says Poland. “Pretty much everyone in the field thinks they’re ten times higher than the official numbers, so you’re probably talking at least 400,000 each year. It’s an incredible burden but because of concerns about anti-vax protestors, until very recently, no manufacturer has wanted to touch this.”
Such was the backlash surrounding the failed LYMErix program that scientists have even explored the most creative of workarounds for protecting people in tick-populated regions, without needing to actually vaccinate them. One research program at the University of Tennessee came up with the idea of leaving food pellets containing a vaccine in woodland areas with the idea that rodents would eat the pellets, and the vaccine would then kill Borrelia bacteria within any ticks which subsequently fed on the animals.
Even the Pfizer-Valneva vaccine has been cautiously designed to try and allay any lingering concerns, two decades after LYMErix. “The concept is the same as the original LYMErix vaccine, but it has been made safer by removing regions that had the potential to induce autoimmunity,” says Embers. “There will always be individuals who oppose vaccines, Lyme or otherwise, but it will be a tremendous boost to public health to have the option.”
Vaccine alternatives
Researchers are also considering alternative immunization approaches in case sufficiently large numbers of people choose to reject any Lyme vaccine which gets approved. Researchers at UMass Chan Medical School have developed an artificially generated antibody, administered via an annual injection, which is capable of killing Borrelia bacteria in the guts of ticks before they can get into the human host.
So far animal studies have shown it to be 100 percent effective, while the scientists have completed a Phase I trial in which they tested it for safety on 48 volunteers in Nebraska. Because this approach provides the antibody directly, rather than triggering the human immune system to produce the antibody like a vaccine would, Embers predicts that it could be a viable alternative for the vaccine hesitant as well as providing an option for immunocompromised individuals who cannot produce enough of their own antibodies.
At the same time, many patient groups still raise concerns over the fact that numerous diagnostic tests for Lyme disease have been reported to have a poor accuracy. Without this, they argue that it is difficult to prove whether vaccines or any other form of immunization actually work. “If the disease is not understood enough to create a more accurate test and a universally accepted treatment protocol, particularly for those who weren’t treated promptly, how can we be sure about the efficacy of a vaccine?” says Natasha Metcalf, co-founder of the organization Lyme Disease UK.
Flory points out that there are so many different types of Borrelia bacteria which cause Lyme disease, that the immunizations being developed may only stop a proportion of cases. In addition, she says that chronic Lyme patients often report a whole myriad of co-infections which remain poorly understood and are likely to also be involved in the disease process.
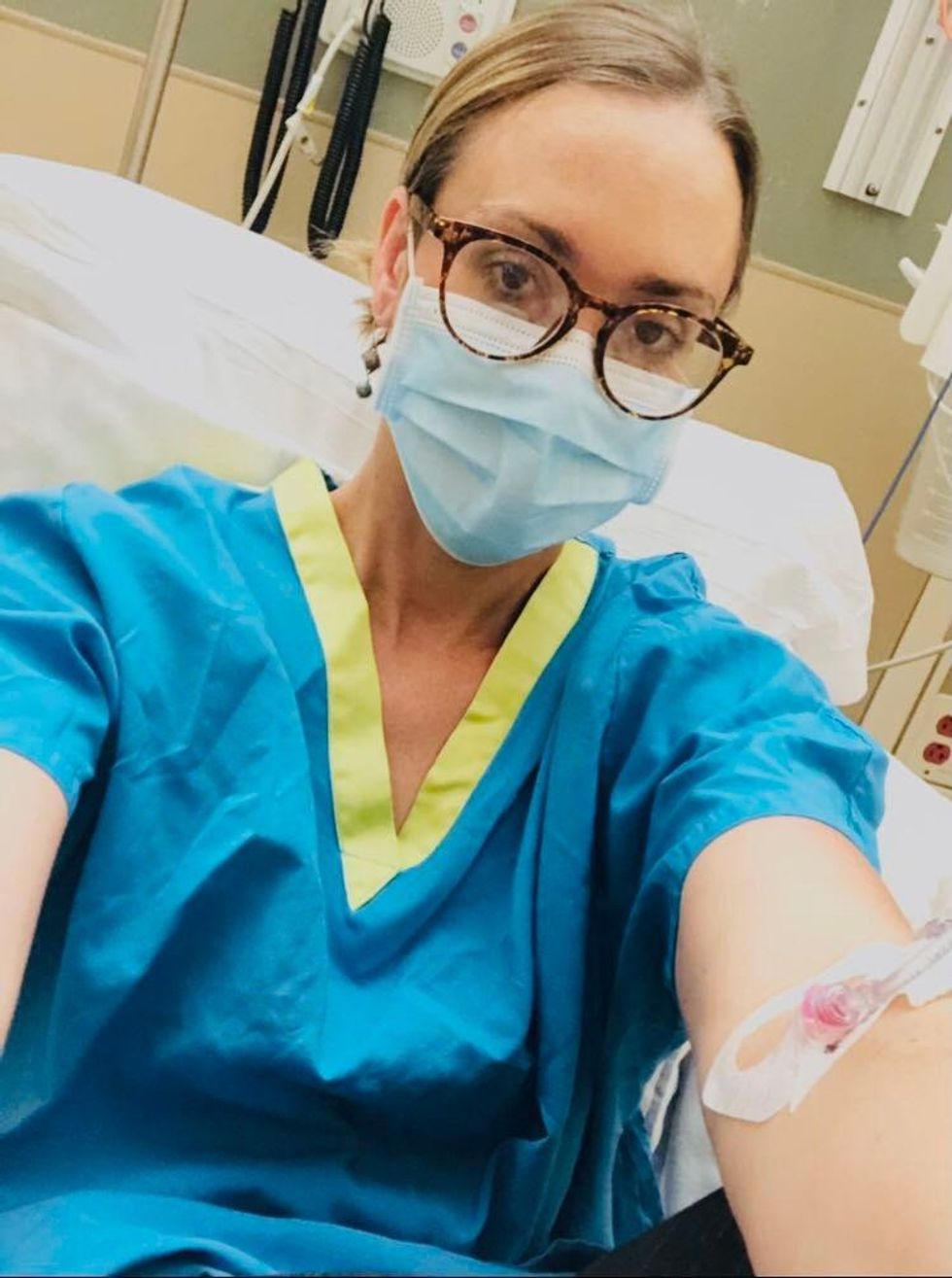
Marci Flory undergoes an infusion in an attempt to treat her Lyme disease symptoms.
Marci Flory
“I would love to see an effective Lyme vaccine but I have my reservations,” she says. “I am infected with four types of Borrelia bacteria, plus many co-infections – Babesia, Bartonella, Erlichiosis, Rickettsia, and Mycoplasma – all from a single Douglas County Kansas tick bite. Lyme never travels alone and the vaccine won’t protect against all the many strains of Borrelia and co-infections.”
Valneva CEO Thomas Lingelbach admits that the Pfizer-Valneva vaccine is not perfect, but predicts that it will still have significant impact if approved.
“We expect the vaccine to have 75 percent plus efficacy,” he says. “There is this legacy around the old Lyme vaccines, but the world is very, very different today. The number of clinical manifestations known to be caused by infection with Lyme Borreliosis has significantly increased, and the understanding around severity has certainly increased.”
Embers agrees that while it will still be important for doctors to monitor for other tick-borne infections which are not necessarily covered by the vaccine, having any clinically approved jab would still represent a major step forward in the fight against the disease.
“I think that any vaccine must be properly vetted, and these companies are performing extensive clinical trials to do just that,” she says. “Lyme is the most common tick-borne disease in the U.S. so the public health impact could be significant. However, clinicians and the general public must remain aware of all of the other tick-borne diseases such as Babesia and Anaplasma, and continue to screen for those when a tick bite is suspected.”
Donna Shirley pictured at her home in Tulsa, with a model of the Sojourner rover she was in charge of that explored Mars.
When NASA's Perseverance rover landed successfully on Mars on February 18, 2021, calling it "one giant leap for mankind" – as Neil Armstrong said when he set foot on the moon in 1969 – would have been inaccurate. This year actually marked the fifth time the U.S. space agency has put a remote-controlled robotic exploration vehicle on the Red Planet. And it was a female engineer named Donna Shirley who broke new ground for women in science as the manager of both the Mars Exploration Program and the 30-person team that built Sojourner, the first rover to land on Mars on July 4, 1997.
For Shirley, the Mars Pathfinder mission was the climax of her 32-year career at NASA's Jet Propulsion Laboratory (JPL) in Pasadena, California. The Oklahoma-born scientist, who earned her Master's degree in aerospace engineering from the University of Southern California, saw her profile skyrocket with media appearances from CNN to the New York Times, and her autobiography Managing Martians came out in 1998. Now 79 and living in a Tulsa retirement community, she still embraces her status as a female pioneer.
"Periodically, I'll hear somebody say they got into the space program because of me, and that makes me feel really good," Shirley told Leaps.org. "I look at the mission control area, and there are a lot of women in there. I'm quite pleased I was able to break the glass ceiling."
Her $25-million, 25-pound microrover – powered by solar energy and designed to get rock samples and test soil chemistry for evidence of life – was named after Sojourner Truth, a 19th-century Black abolitionist and women's rights activist. Unlike Mars Pathfinder, Shirley didn't have to travel more than 131 million miles to reach her goal, but her path to scientific fame as a woman sometimes resembled an asteroid field.
 The Sojourner Rover in 1997 on Mars.NASA/JPL
The Sojourner Rover in 1997 on Mars.NASA/JPLAs a high-IQ tomboy growing up in Wynnewood, Oklahoma (pop. 2,300), Shirley yearned to escape. She decided to become an engineer at age 10 and took flying lessons at 15. Her extraterrestrial aspirations were fueled by Ray Bradbury's The Martian Chronicles and Arthur C. Clarke's The Sands of Mars. Yet when she entered the University of Oklahoma (OU) in 1958, her freshman academic advisor initially told her: "Girls can't be engineers." She ignored him.
Years later, Shirley would combat such archaic thinking, succeeding at JPL with her creative, collaborative management style. "If you look at the literature, you'll find that teams that are either led by or heavily involved with women do better than strictly male teams," she noted.
However, her career trajectory stalled at OU. Burned out by her course load and distracted by a broken engagement to marry a fellow student, she switched her major to professional writing. After graduation, she applied her aeronautical background as a McDonnell Aircraft technical writer, but her boss, she says, harassed her and she faced gender-based hostility from male co-workers.
Returning to OU, Shirley finished off her engineering degree and became a JPL aerodynamist in 1966 after answering an ad in the St. Louis Post-Dispatch. At first, she was the only female engineer among the research center's 2,000-odd engineers. She wore many hats, from designing planetary atmospheric entry vehicles to picking the launch date of November 4, 1973 for Mariner 10's mission to Venus and Mercury.
By the mid-1980's, she was managing teams that focused on robotics and Mars, delivering creative solutions when NASA budget cuts loomed. In 1989, the same year the Sojourner microrover concept was born, President George H.W. Bush announced his Space Exploration Initiative, including plans for a human mission to Mars by 2019.
That target, of course, wasn't attained, despite huge advances in technology and our understanding of the Martian environment. Today, Shirley believes humans could land on Mars by 2030. She became the founding director of the Science Fiction Museum and Hall of Fame in Seattle in 2004 after leaving NASA, and to this day, she enjoys checking out pop culture portrayals of Mars landings – even if they're not always accurate.
After the novel The Martian was published in 2011, which later was adapted into the hit film starring Matt Damon, Shirley phoned author Andy Weir: "You've got a major mistake in here. It says there's a storm that tries to blow the rocket over. But actually, the Mars atmosphere is so thin, it would never blow a rocket over!"
Fearlessly speaking her mind and seeking the stars helped Donna Shirley make history. However, a 2019 Washington Post story noted: "Women make up only about a third of NASA's workforce. They comprise just 28 percent of senior executive leadership positions and are only 16 percent of senior scientific employees." Whether it's traveling to Mars or trending toward gender equality, we've still got a long way to go.
Announcing March Event: "COVID Vaccines and the Return to Life: Part 1"
Leading medical and scientific experts will discuss the latest developments around the COVID-19 vaccines at our March 11th event.
EVENT INFORMATION
DATE:
Thursday, March 11th, 2021 at 12:30pm - 1:45pm EST
On the one-year anniversary of the global declaration of the pandemic, this virtual event will convene leading scientific and medical experts to discuss the most pressing questions around the COVID-19 vaccines. Planned topics include the effect of the new circulating variants on the vaccines, what we know so far about transmission dynamics post-vaccination, how individuals can behave post-vaccination, the myths of "good" and "bad" vaccines as more alternatives come on board, and more. A public Q&A will follow the expert discussion.
CONTACT:
kira@goodinc.com
LOCATION:
Zoom webinar
SPEAKERS:

Dr. Paul Offit speaking at Communicating Vaccine Science.
commons.wikimedia.orgDr. Paul Offit, M.D., is the director of the Vaccine Education Center and an attending physician in infectious diseases at the Children's Hospital of Philadelphia. He is a co-inventor of the rotavirus vaccine for infants, and he has lent his expertise to the advisory committees that review data on new vaccines for the CDC and FDA.

Dr. Monica Gandhi
UCSF Health
Dr. Monica Gandhi, M.D., MPH, is Professor of Medicine and Associate Division Chief (Clinical Operations/ Education) of the Division of HIV, Infectious Diseases, and Global Medicine at UCSF/ San Francisco General Hospital.

Dr. Onyema Ogbuagu, MBBCh, FACP, FIDSA
Yale Medicine
Dr. Onyema Ogbuagu, MBBCh, is an infectious disease physician at Yale Medicine who treats COVID-19 patients and leads Yale's clinical studies around COVID-19. He ran Yale's trial of the Pfizer/BioNTech vaccine.

Dr. Eric Topol
Dr. Topol's Twitter
Dr. Eric Topol, M.D., is a cardiologist, scientist, professor of molecular medicine, and the director and founder of Scripps Research Translational Institute. He has led clinical trials in over 40 countries with over 200,000 patients and pioneered the development of many routinely used medications.
REGISTER NOW
This event is the first of a four-part series co-hosted by LeapsMag, the Aspen Institute Science & Society Program, and the Sabin–Aspen Vaccine Science & Policy Group, with generous support from the Gordon and Betty Moore Foundation and the Howard Hughes Medical Institute.

Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.