A vaccine for Lyme disease could be coming. But will patients accept it?

Hiking is one of Marci Flory's favorite activities, but her chronic Lyme disease sometimes renders her unable to walk. Biotech companies Pfizer and Valneva are testing a vaccine for Lyme disease in a Phase III clinical trial.
For more than two decades, Marci Flory, a 40-year-old emergency room nurse from Lawrence, Kan., has battled the recurring symptoms of chronic Lyme disease, an illness which she believes began after being bitten by a tick during her teenage years.
Over the years, Flory has been plagued by an array of mysterious ailments, ranging from fatigue to crippling pain in her eyes, joints and neck, and even postural tachycardia syndrome or PoTS, an abnormal increase in heart rate after sitting up or standing. Ten years ago, she began to experience the onset of neurological symptoms which ranged from brain fog to sudden headaches, and strange episodes of leg weakness which would leave her unable to walk.
“Initially doctors thought I had ALS, or less likely, multiple sclerosis,” she says. “But after repeated MRI scans for a year, they concluded I had a rare neurological condition called acute transverse myelitis.”
But Flory was not convinced. After ordering a variety of private blood tests, she discovered she was infected with a range of bacteria in the genus Borrelia that live in the guts of ticks, the infectious agents responsible for Lyme disease.
“It made sense,” she says. “Looking back, I was bitten in high school and misdiagnosed with mononucleosis. This was probably the start, and my immune system kept it under wraps for a while. The Lyme bacteria can burrow into every tissue in the body, go into cyst form and become dormant before reactivating.”
The reason why cases of Lyme disease are increasing is down to changing weather patterns, triggered by climate change, meaning that ticks are now found across a much wider geographic range than ever before.
When these species of bacteria are transmitted to humans, they can attack the nervous system, joints and even internal organs which can lead to serious health complications such as arthritis, meningitis and even heart failure. While Lyme disease can sometimes be successfully treated with antibiotics if spotted early on, not everyone responds to these drugs, and for patients who have developed chronic symptoms, there is no known cure. Flory says she knows of fellow Lyme disease patients who have spent hundreds of thousands of dollars seeking treatments.
Concerningly, statistics show that Lyme and other tick-borne diseases are on the rise. Recently released estimates based on health insurance records suggest that at least 476,000 Americans are diagnosed with Lyme disease every year, and many experts believe the true figure is far higher.
The reason why the numbers are growing is down to changing weather patterns, triggered by climate change, meaning that ticks are now found across a much wider geographic range than ever before. Health insurance data shows that cases of Lyme disease have increased fourfold in rural parts of the U.S. over the last 15 years, and 65 percent in urban regions.
As a result, many scientists who have studied Lyme disease feel that it is paramount to bring some form of protective vaccine to market which can be offered to people living in the most at-risk areas.
“Even the increased awareness for Lyme disease has not stopped the cases,” says Eva Sapi, professor of cellular and molecular biology at the University of New Haven. “Some of these patients are looking for answers for years, running from one doctor to another, so that is obviously a very big cost for our society at so many levels.”
Emerging vaccines – and backlash
But with the rising case numbers, interest has grown among the pharmaceutical industry and research communities. Vienna-based biotech Valneva have partnered with Pfizer to take their vaccine – a seasonal jab which offers protection against the six most common strains of Lyme disease in the northern hemisphere – into a Phase III clinical trial which began in August. Involving 6,000 participants in a number of U.S. states and northern Europe where Lyme disease is endemic, it could lead to a licensed vaccine by 2025, if it proves successful.
“For many years Lyme was considered a small market vaccine,” explains Monica E. Embers, assistant professor of parasitology at Tulane University in New Orleans. “Now we know that this is a much bigger problem, Pfizer has stepped up to invest in preventing this disease and other pharmaceutical companies may as well.”
Despite innovations, patient communities and their representatives remain ambivalent about the idea of a vaccine. Some of this skepticism dates back to the failed LYMErix vaccine which was developed in the late 1990s before being withdrawn from the market.
At the same time, scientists at Yale University are developing a messenger RNA vaccine which aims to train the immune system to respond to tick bites by exposing it to 19 proteins found in tick saliva. Whereas the Valneva vaccine targets the bacteria within ticks, the Yale vaccine attempts to provoke an instant and aggressive immune response at the site of the bite. This causes the tick to fall off and limits the potential for transmitting dangerous infections.
But despite these innovations, patient communities and their representatives remain ambivalent about the idea of a vaccine. Some of this skepticism dates back to the failed LYMErix vaccine which was developed in the late 1990s before being withdrawn from the market in 2002 after concerns were raised that it might induce autoimmune reactions in humans.
While this theory was ultimately disproved, the lingering stigma attached to LYMErix meant that most vaccine manufacturers chose to stay away from the disease for many years, something which Gregory Poland, head of the Mayo Clinic’s Vaccine Research Group in Minnesota, describes as a tragedy.
“Since 2002, we have not had a human Lyme vaccine in the U.S. despite the increasing number of cases,” says Poland. “Pretty much everyone in the field thinks they’re ten times higher than the official numbers, so you’re probably talking at least 400,000 each year. It’s an incredible burden but because of concerns about anti-vax protestors, until very recently, no manufacturer has wanted to touch this.”
Such was the backlash surrounding the failed LYMErix program that scientists have even explored the most creative of workarounds for protecting people in tick-populated regions, without needing to actually vaccinate them. One research program at the University of Tennessee came up with the idea of leaving food pellets containing a vaccine in woodland areas with the idea that rodents would eat the pellets, and the vaccine would then kill Borrelia bacteria within any ticks which subsequently fed on the animals.
Even the Pfizer-Valneva vaccine has been cautiously designed to try and allay any lingering concerns, two decades after LYMErix. “The concept is the same as the original LYMErix vaccine, but it has been made safer by removing regions that had the potential to induce autoimmunity,” says Embers. “There will always be individuals who oppose vaccines, Lyme or otherwise, but it will be a tremendous boost to public health to have the option.”
Vaccine alternatives
Researchers are also considering alternative immunization approaches in case sufficiently large numbers of people choose to reject any Lyme vaccine which gets approved. Researchers at UMass Chan Medical School have developed an artificially generated antibody, administered via an annual injection, which is capable of killing Borrelia bacteria in the guts of ticks before they can get into the human host.
So far animal studies have shown it to be 100 percent effective, while the scientists have completed a Phase I trial in which they tested it for safety on 48 volunteers in Nebraska. Because this approach provides the antibody directly, rather than triggering the human immune system to produce the antibody like a vaccine would, Embers predicts that it could be a viable alternative for the vaccine hesitant as well as providing an option for immunocompromised individuals who cannot produce enough of their own antibodies.
At the same time, many patient groups still raise concerns over the fact that numerous diagnostic tests for Lyme disease have been reported to have a poor accuracy. Without this, they argue that it is difficult to prove whether vaccines or any other form of immunization actually work. “If the disease is not understood enough to create a more accurate test and a universally accepted treatment protocol, particularly for those who weren’t treated promptly, how can we be sure about the efficacy of a vaccine?” says Natasha Metcalf, co-founder of the organization Lyme Disease UK.
Flory points out that there are so many different types of Borrelia bacteria which cause Lyme disease, that the immunizations being developed may only stop a proportion of cases. In addition, she says that chronic Lyme patients often report a whole myriad of co-infections which remain poorly understood and are likely to also be involved in the disease process.
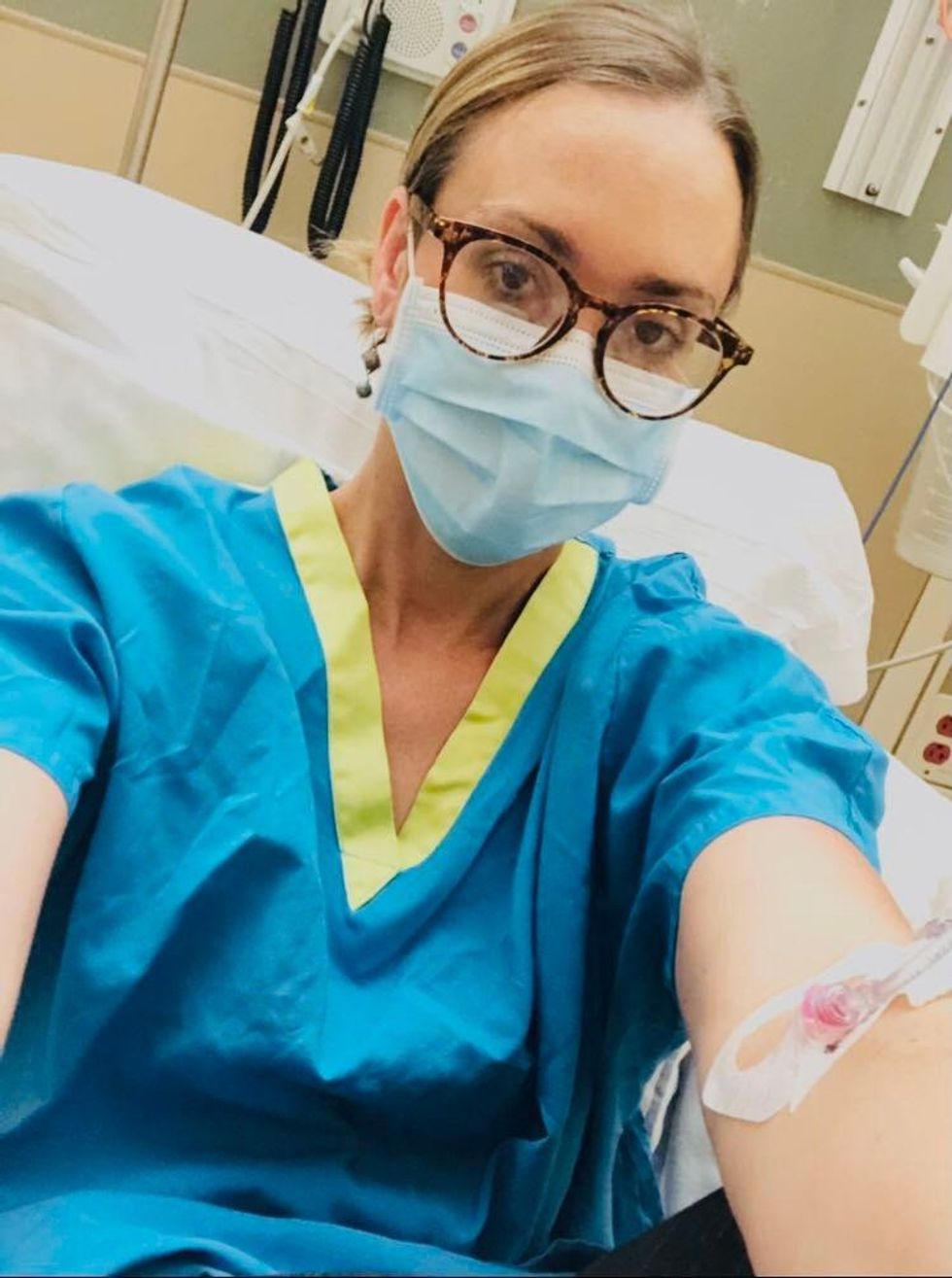
Marci Flory undergoes an infusion in an attempt to treat her Lyme disease symptoms.
Marci Flory
“I would love to see an effective Lyme vaccine but I have my reservations,” she says. “I am infected with four types of Borrelia bacteria, plus many co-infections – Babesia, Bartonella, Erlichiosis, Rickettsia, and Mycoplasma – all from a single Douglas County Kansas tick bite. Lyme never travels alone and the vaccine won’t protect against all the many strains of Borrelia and co-infections.”
Valneva CEO Thomas Lingelbach admits that the Pfizer-Valneva vaccine is not perfect, but predicts that it will still have significant impact if approved.
“We expect the vaccine to have 75 percent plus efficacy,” he says. “There is this legacy around the old Lyme vaccines, but the world is very, very different today. The number of clinical manifestations known to be caused by infection with Lyme Borreliosis has significantly increased, and the understanding around severity has certainly increased.”
Embers agrees that while it will still be important for doctors to monitor for other tick-borne infections which are not necessarily covered by the vaccine, having any clinically approved jab would still represent a major step forward in the fight against the disease.
“I think that any vaccine must be properly vetted, and these companies are performing extensive clinical trials to do just that,” she says. “Lyme is the most common tick-borne disease in the U.S. so the public health impact could be significant. However, clinicians and the general public must remain aware of all of the other tick-borne diseases such as Babesia and Anaplasma, and continue to screen for those when a tick bite is suspected.”
Kids' immune systems come into contact with so many antigens every day that extra cleaning and disinfecting won't harm them, experts say.
Cleaning has taken on a whole new meaning in Frank Mosco's household during the COVID-19 pandemic. There's a protocol for everything he and his two teenage daughters do.
Experts agree that over-disinfecting is better than inadequate disinfecting, especially during a pandemic.
"We wipe down every package that comes into the house and the items inside," says Mosco, a technologist and social justice activist in Hastings-on-Hudson, N.Y. "If it's a fruit or vegetable, I use vinegar and water, or water and soap. Then we throw out the boxes, clean up the table, and wash our hands." Only then do they put items away.
As the novel coronavirus continues to pose an invisible threat, parents of infants to adolescents are pondering how vigorously and frequently to clean and disinfect surfaces at home and apply hand sanitizer in public. They also fret over whether there can be too much of a good thing: Will making everything as seemingly germ-free as possible reduce immunity down the road?
Experts agree that over-disinfecting is better than inadequate disinfecting, especially during a pandemic. Every family should assess their particular risks. Factors to consider include pre-existing medical conditions, the number of people living in the same home, and whether anyone works in a hospital or other virus-prone environment, says Kari Debbink, assistant professor of biology at Bowie State University in Bowie, Maryland.
Constantly cleaning everything in sight isn't necessary, she explains, because coronavirus tends to spread mainly via immediate contact with respiratory droplets—catching it from surfaces is a less-likely scenario. The longer the virus stays on a surface, the less contagious it becomes.
Some parents worry that their children's growing bodies may become accustomed to an environment that is "too clean." Debbink, a virologist, offers a salient reminder: "The immune system comes into contact with many, many different antigens every day, and it is 'trained' from birth onwards to respond to pathogens. Doing a little more cleansing and disinfecting during the pandemic will not weaken the immune system."
Other experts agree. "There should be no negative outcome to properly washing your hands more frequently," says Stacey Schultz-Cherry, an infectious diseases specialist at St. Jude Children's Research Hospital in Memphis, Tennessee. "Even with enhanced disinfection, kids are still getting exposed to immune-boosting microbes from playing outside, having pets, etc."
"There's no reason why hand sanitizer would weaken anyone's immune system of any age."
Applying hand sanitizer consisting of at least 60 percent alcohol helps clean hands while outdoors, says Angela Rasmussen, associate research scientist and a virologist at Columbia University's Mailman School of Public Health in New York. "There's no reason why hand sanitizer would weaken anyone's immune system of any age," she adds, and recommends moisturizer so hands don't dry out from frequent use. Meanwhile, "cleaning and disinfecting at home also don't have an impact on antiviral immunity, in kids or adults."
With the coronavirus foremost in parents' minds, Patricia Garcia, a pediatric hospitalist, has fielded many questions about how thoroughly they should wipe, rub, scrub, or mop. As medical director of Connecticut Children's Healthy Homes Program in Hartford, which takes aim at toxins and other housing hazards, she reassures them with this mantra: "You're never going to get it perfectly sterilized, and that's okay."
To quell some of these concerns, in March the U.S. Environmental Protection Agency (EPA) released a list of products for household use. None of these products have been specifically tested against SARS-CoV-2, the novel coronavirus that causes COVID-19. But the agency expects these products to be effective because they have demonstrated efficacy against a different human coronavirus similar to SARS-CoV-2 or an even harder-to-kill virus.
Many products on the list contain isopropyl alcohol or hydrogen peroxide. "When using an EPA-registered disinfectant," the agency's website instructs, "follow the label directions for safe, effective use. Make sure to follow the contact time, which is the amount of time the surface should be visibly wet."
Bear in mind that not all cleaners actually disinfect, cautions Alan Woolf, a pediatrician at Boston Children's Hospital who directs its environmental health center and is a professor at Harvard Medical School. Some cleaners remove visible dirt, grease, and grime, but they don't kill viruses. Disinfectants by their nature inactivate both bacteria and viruses. "That's an important distinction," Woolf says.
Frequently touched surfaces—for instance, doorknobs, light switches, toilet-flushing levers, and countertops—should not only be cleaned, but also disinfected at least daily during a pandemic if someone in the household is sick. The objects one touches upon coming home are the ones most likely to become contaminated with viruses, experts say.
Before bringing items inside, "it might be good to clear off a counter space where they will be placed," says Debbink, the biology professor and virologist. "This way, they come into contact with as few items and surfaces as possible."
If space permits, another option would be to set aside nonperishable items. "I've heard of some families putting things in a 'mud room' and closing the door for 48 hours, some leaving things in their garage or car trunk," says Stephanie Holm, co-director of the Western States Pediatric Environmental Health Specialty Unit at the University of California, San Francisco. "Letting new purchases sit for 48 hours undisturbed would greatly reduce the number of viable viruses present."
Cleaning surfaces is recommended before disinfecting them. Holm suggests using unscented soap and microfiber cloths instead of paper towels, which can transmit bacteria and viruses from one area to another.
Soap has the power to eradicate viruses with at least 20 seconds of contact time. It attacks the coronavirus's protective coat, explains infectious diseases specialist Schultz-Cherry. "If you destroy the coat, the virus is no longer infectious. Influenza virus is also very sensitive to soap."
"The most important thing that parents should do for children's immune systems is make sure they are up to date on all their vaccines."
For cribs, toys, and other mouth-contact surfaces, sanitizing with soap and water, not disinfectants, is advisable, says pediatrician Woolf. Fresh fruits and vegetables also can be cleaned with soap, removing dirt and pesticide residue, he adds.
"Some parents are nervous about using disinfectant on toys, which is understandable, considering many toys end up in children's mouths, so soap and water can be an alternative," says pediatrician Garcia, who recommends using hot water.
While some toys can go in the washing machine and dryer or dishwasher, others need to be cleaned by hand, with dish soap or a delicate detergent, as indicated on their labels. But toys with electrical components cannot be submerged in water, in which case consulting the EPA's list of disinfectants may be a parent's best option, she says.
Labels on the back of cleaning and disinfecting products also contain specific instructions. Not allowing a liquid to sit on a surface for the recommended time results in exposure to chemicals without even accomplishing the intended purpose of disinfection. For most household bleach-containing agents, the advisable "dwell time" is 10 minutes. "Many people don't realize this," says Holm, the environmental health specialist who also trained as a physician.
Beware of combining any type of cleaners or disinfectants that aren't already premixed. Doing so can release harmful gases into the air, she cautions.
During the pandemic, Mosco and his daughters have been very conscientious about decontaminating whatever comes through their doors. Mosco says he doesn't believe the family is overusing cleaning and disinfecting products. Although he's fastidious, he says, "a completely sterile environment is not the goal."
His mother, who was a nurse, instilled in him that exposure to some bacteria is a good thing. In turn, he "always encouraged his kids to play with animals, and to have fun in sand and dirt, with plenty of sunlight to keep their immune systems strong."
Even though a vaccine for coronavirus currently doesn't exist, parents can take some comfort in the best weapon available today to protect kids from deadly pathogens: "The most important thing that parents should do for children's immune systems," says virologist Rasmussen, "is make sure they are up to date on all their vaccines."
A researcher works in the lab at Alnylam Pharmaceuticals, which has pioneered the development of RNAi therapies.
In October 2006, Craig Mello received a strange phone call from Sweden at 4:30 a.m. The voice at the other end of the line told him to get dressed and that his life was about to change.
"We think this could be effective in [the early] phase, helping the body clear the virus and preventing progression to that severe hyperimmune response which occurs in some patients."
Shortly afterwards, he was informed that along with his colleague Andrew Fire, he had won the Nobel Prize in Physiology or Medicine.
Eight years earlier, biologists Fire and Mello had made a landmark discovery in the history of genetics. In a series of experiments conducted in worms, they had revealed an ancient evolutionary mechanism present in all animals that allows RNA – the structures within our cells that take genetic information from DNA and use it to make proteins – to selectively switch off genes.
At the time, scientists heralded the dawn of a new field of medical research utilizing this mechanism, known as RNA interference or RNAi, to tackle rare genetic diseases and deactivate viruses. Now, 14 years later, the pharmaceutical company Alnylam — which has pioneered the development of RNAi-based treatments over the past decade — is looking to use it to develop a groundbreaking drug for the virus that causes COVID-19.
"We can design small interfering RNAs to target regions of the viral genome and bind to them," said Akin Akinc, who manages several of Alnylam's drug development programs. "What we're learning about COVID-19 is that there's an early phase where there's lots of viral replication and a high viral load. We think this could be effective in that phase, helping the body clear the virus and preventing progression to that severe hyperimmune response which occurs in some patients."
Called ALN-COV, Alnylam's treatment hypothetically works by switching off a key gene in the virus, inhibiting its ability to replicate itself. In order to deliver it to the epithelial cells deep in the lung tissue, where the virus resides, patients will inhale a fine mist containing the RNAi molecules mixed in a saline solution, using a nebulizer.
But before human trials of the drug can begin, the company needs to convince regulators that it is both safe and effective in a series of preclinical trials. While early results appear promising - when mixed with the virus in a test tube, the drug displayed a 95 percent inhibition rate – experts are reserving judgment until it performs in clinical trials.
"If successful this could be a very important milestone in the development of RNAi therapies, but virus infections are very complicated and it can be hard to predict whether a given level of inhibition in cell culture will be sufficient to have a significant impact on the course of the infection," said Si-Ping Han, who researches RNAi therapeutics at California Institute of Technology and is not involved in the development of this drug.
So far, Alnylam has had success in using RNAi to treat rare genetic diseases. It currently has treatments licensed for Hereditary ATTR Amyloidosis and Acute Hepatic Porphyria. Another treatment, for Primary Hyperoxaluria Type 1, is currently under regulatory review. But its only previous attempt to use RNAi to tackle a respiratory infection was a failed effort to develop a drug for respiratory syncytial virus (RSV) almost a decade ago.
However, the technology has advanced considerably since then. "Back then, RNAi drugs had no chemical modifications whatsoever, so they were readily degraded by the body, and they could also result in unintended immune stimulation," said Akinc. "Since then, we've learned how to chemically modify our RNAi's to make them immunosilent and give them improved potency, stability, and duration of action."
"It would be a very important milestone in the development of RNAi therapies."
But one key challenge the company will face is the sheer speed at which viruses evolve, meaning they can become drug-resistant very quickly. Scientists predict that Alnylam will ultimately have to develop a series of RNAi drugs for the coronavirus that work together.
"There's been considerable interest in using RNAi to treat viral infections, as RNA therapies can be developed more rapidly than protein therapies like monoclonal antibodies, since one only needs to know the viral genome sequence to begin to design them," said David Schaffer, professor of bioengineering at University of California, Berkeley. "But viruses can evolve their sequences rapidly around single drugs so it is likely that a combinatorial RNAi therapy may be needed."
In the meantime, Alnylam is conducting further preclinical trials over the summer and fall, with the aim of launching testing in human volunteers by the end of this year -- an ambitious aim that would represent a breakneck pace for a drug development program.
If the approach does ultimately succeed, it would represent a major breakthrough for the field as a whole, potentially opening the door to a whole new wave of RNAi treatments for different lung infections and diseases.
"It would be a very important milestone in the development of RNAi therapies," said Han, the Caltech researcher. "It would be both the first time that an RNAi drug has been successfully used to treat a respiratory infection and as far as I know, the first time that one has been successful in treating any disease in the lungs. RNAi is a platform that can be reconfigured to hit different targets, and so once the first drug has been developed, we can expect a rapid flow of variants targeting other respiratory infections or other lung diseases."

