A vaccine for Lyme disease could be coming. But will patients accept it?

Hiking is one of Marci Flory's favorite activities, but her chronic Lyme disease sometimes renders her unable to walk. Biotech companies Pfizer and Valneva are testing a vaccine for Lyme disease in a Phase III clinical trial.
For more than two decades, Marci Flory, a 40-year-old emergency room nurse from Lawrence, Kan., has battled the recurring symptoms of chronic Lyme disease, an illness which she believes began after being bitten by a tick during her teenage years.
Over the years, Flory has been plagued by an array of mysterious ailments, ranging from fatigue to crippling pain in her eyes, joints and neck, and even postural tachycardia syndrome or PoTS, an abnormal increase in heart rate after sitting up or standing. Ten years ago, she began to experience the onset of neurological symptoms which ranged from brain fog to sudden headaches, and strange episodes of leg weakness which would leave her unable to walk.
“Initially doctors thought I had ALS, or less likely, multiple sclerosis,” she says. “But after repeated MRI scans for a year, they concluded I had a rare neurological condition called acute transverse myelitis.”
But Flory was not convinced. After ordering a variety of private blood tests, she discovered she was infected with a range of bacteria in the genus Borrelia that live in the guts of ticks, the infectious agents responsible for Lyme disease.
“It made sense,” she says. “Looking back, I was bitten in high school and misdiagnosed with mononucleosis. This was probably the start, and my immune system kept it under wraps for a while. The Lyme bacteria can burrow into every tissue in the body, go into cyst form and become dormant before reactivating.”
The reason why cases of Lyme disease are increasing is down to changing weather patterns, triggered by climate change, meaning that ticks are now found across a much wider geographic range than ever before.
When these species of bacteria are transmitted to humans, they can attack the nervous system, joints and even internal organs which can lead to serious health complications such as arthritis, meningitis and even heart failure. While Lyme disease can sometimes be successfully treated with antibiotics if spotted early on, not everyone responds to these drugs, and for patients who have developed chronic symptoms, there is no known cure. Flory says she knows of fellow Lyme disease patients who have spent hundreds of thousands of dollars seeking treatments.
Concerningly, statistics show that Lyme and other tick-borne diseases are on the rise. Recently released estimates based on health insurance records suggest that at least 476,000 Americans are diagnosed with Lyme disease every year, and many experts believe the true figure is far higher.
The reason why the numbers are growing is down to changing weather patterns, triggered by climate change, meaning that ticks are now found across a much wider geographic range than ever before. Health insurance data shows that cases of Lyme disease have increased fourfold in rural parts of the U.S. over the last 15 years, and 65 percent in urban regions.
As a result, many scientists who have studied Lyme disease feel that it is paramount to bring some form of protective vaccine to market which can be offered to people living in the most at-risk areas.
“Even the increased awareness for Lyme disease has not stopped the cases,” says Eva Sapi, professor of cellular and molecular biology at the University of New Haven. “Some of these patients are looking for answers for years, running from one doctor to another, so that is obviously a very big cost for our society at so many levels.”
Emerging vaccines – and backlash
But with the rising case numbers, interest has grown among the pharmaceutical industry and research communities. Vienna-based biotech Valneva have partnered with Pfizer to take their vaccine – a seasonal jab which offers protection against the six most common strains of Lyme disease in the northern hemisphere – into a Phase III clinical trial which began in August. Involving 6,000 participants in a number of U.S. states and northern Europe where Lyme disease is endemic, it could lead to a licensed vaccine by 2025, if it proves successful.
“For many years Lyme was considered a small market vaccine,” explains Monica E. Embers, assistant professor of parasitology at Tulane University in New Orleans. “Now we know that this is a much bigger problem, Pfizer has stepped up to invest in preventing this disease and other pharmaceutical companies may as well.”
Despite innovations, patient communities and their representatives remain ambivalent about the idea of a vaccine. Some of this skepticism dates back to the failed LYMErix vaccine which was developed in the late 1990s before being withdrawn from the market.
At the same time, scientists at Yale University are developing a messenger RNA vaccine which aims to train the immune system to respond to tick bites by exposing it to 19 proteins found in tick saliva. Whereas the Valneva vaccine targets the bacteria within ticks, the Yale vaccine attempts to provoke an instant and aggressive immune response at the site of the bite. This causes the tick to fall off and limits the potential for transmitting dangerous infections.
But despite these innovations, patient communities and their representatives remain ambivalent about the idea of a vaccine. Some of this skepticism dates back to the failed LYMErix vaccine which was developed in the late 1990s before being withdrawn from the market in 2002 after concerns were raised that it might induce autoimmune reactions in humans.
While this theory was ultimately disproved, the lingering stigma attached to LYMErix meant that most vaccine manufacturers chose to stay away from the disease for many years, something which Gregory Poland, head of the Mayo Clinic’s Vaccine Research Group in Minnesota, describes as a tragedy.
“Since 2002, we have not had a human Lyme vaccine in the U.S. despite the increasing number of cases,” says Poland. “Pretty much everyone in the field thinks they’re ten times higher than the official numbers, so you’re probably talking at least 400,000 each year. It’s an incredible burden but because of concerns about anti-vax protestors, until very recently, no manufacturer has wanted to touch this.”
Such was the backlash surrounding the failed LYMErix program that scientists have even explored the most creative of workarounds for protecting people in tick-populated regions, without needing to actually vaccinate them. One research program at the University of Tennessee came up with the idea of leaving food pellets containing a vaccine in woodland areas with the idea that rodents would eat the pellets, and the vaccine would then kill Borrelia bacteria within any ticks which subsequently fed on the animals.
Even the Pfizer-Valneva vaccine has been cautiously designed to try and allay any lingering concerns, two decades after LYMErix. “The concept is the same as the original LYMErix vaccine, but it has been made safer by removing regions that had the potential to induce autoimmunity,” says Embers. “There will always be individuals who oppose vaccines, Lyme or otherwise, but it will be a tremendous boost to public health to have the option.”
Vaccine alternatives
Researchers are also considering alternative immunization approaches in case sufficiently large numbers of people choose to reject any Lyme vaccine which gets approved. Researchers at UMass Chan Medical School have developed an artificially generated antibody, administered via an annual injection, which is capable of killing Borrelia bacteria in the guts of ticks before they can get into the human host.
So far animal studies have shown it to be 100 percent effective, while the scientists have completed a Phase I trial in which they tested it for safety on 48 volunteers in Nebraska. Because this approach provides the antibody directly, rather than triggering the human immune system to produce the antibody like a vaccine would, Embers predicts that it could be a viable alternative for the vaccine hesitant as well as providing an option for immunocompromised individuals who cannot produce enough of their own antibodies.
At the same time, many patient groups still raise concerns over the fact that numerous diagnostic tests for Lyme disease have been reported to have a poor accuracy. Without this, they argue that it is difficult to prove whether vaccines or any other form of immunization actually work. “If the disease is not understood enough to create a more accurate test and a universally accepted treatment protocol, particularly for those who weren’t treated promptly, how can we be sure about the efficacy of a vaccine?” says Natasha Metcalf, co-founder of the organization Lyme Disease UK.
Flory points out that there are so many different types of Borrelia bacteria which cause Lyme disease, that the immunizations being developed may only stop a proportion of cases. In addition, she says that chronic Lyme patients often report a whole myriad of co-infections which remain poorly understood and are likely to also be involved in the disease process.
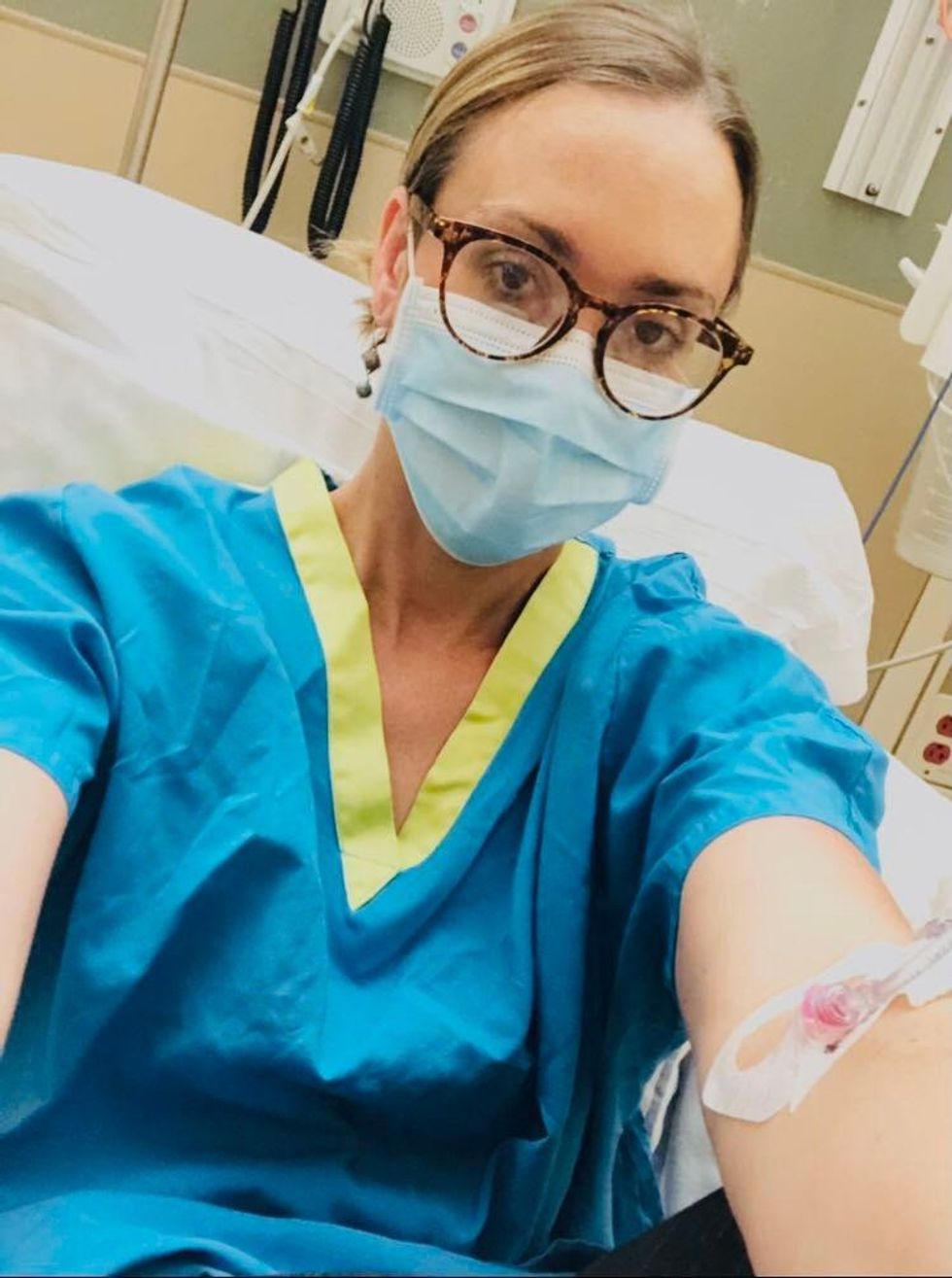
Marci Flory undergoes an infusion in an attempt to treat her Lyme disease symptoms.
Marci Flory
“I would love to see an effective Lyme vaccine but I have my reservations,” she says. “I am infected with four types of Borrelia bacteria, plus many co-infections – Babesia, Bartonella, Erlichiosis, Rickettsia, and Mycoplasma – all from a single Douglas County Kansas tick bite. Lyme never travels alone and the vaccine won’t protect against all the many strains of Borrelia and co-infections.”
Valneva CEO Thomas Lingelbach admits that the Pfizer-Valneva vaccine is not perfect, but predicts that it will still have significant impact if approved.
“We expect the vaccine to have 75 percent plus efficacy,” he says. “There is this legacy around the old Lyme vaccines, but the world is very, very different today. The number of clinical manifestations known to be caused by infection with Lyme Borreliosis has significantly increased, and the understanding around severity has certainly increased.”
Embers agrees that while it will still be important for doctors to monitor for other tick-borne infections which are not necessarily covered by the vaccine, having any clinically approved jab would still represent a major step forward in the fight against the disease.
“I think that any vaccine must be properly vetted, and these companies are performing extensive clinical trials to do just that,” she says. “Lyme is the most common tick-borne disease in the U.S. so the public health impact could be significant. However, clinicians and the general public must remain aware of all of the other tick-borne diseases such as Babesia and Anaplasma, and continue to screen for those when a tick bite is suspected.”
A Mother-and-Daughter Team Have Developed What May Be the World’s First Alzheimer’s Vaccine
Brain inflammation from Alzheimer's disease.
Alzheimer's is a terrible disease that robs a person of their personality and memory before eventually leading to death. It's the sixth-largest killer in the U.S. and, currently, there are 5.8 million Americans living with the disease.
Wang's vaccine is a significant improvement over previous attempts because it can attack the Alzheimer's protein without creating any adverse side effects.
It devastates people and families and it's estimated that Alzheimer's and other forms of dementia will cost the U.S. $290 billion dollars this year alone. It's estimated that it will become a trillion-dollar-a-year disease by 2050.
There have been over 200 unsuccessful attempts to find a cure for the disease and the clinical trial termination rate is 98 percent.
Alzheimer's is caused by plaque deposits that develop in brain tissue that become toxic to brain cells. One of the major hurdles to finding a cure for the disease is that it's impossible to clear out the deposits from the tissue. So scientists have turned their attention to early detection and prevention.
One very encouraging development has come out of the work done by Dr. Chang Yi Wang, PhD. Wang is a prolific bio-inventor; one of her biggest successes is developing a foot-and-mouth vaccine for pigs that has been administered more than three billion times.

Mei Mei Hu
Brainstorm Health / Flickr.
In January, United Neuroscience, a biotech company founded by Yi, her daughter Mei Mei Hu, and son-in-law, Louis Reese, announced the first results from a phase IIa clinical trial on UB-311, an Alzheimer's vaccine.
The vaccine has synthetic versions of amino acid chains that trigger antibodies to attack Alzheimer's protein the blood. Wang's vaccine is a significant improvement over previous attempts because it can attack the Alzheimer's protein without creating any adverse side effects.
"We were able to generate some antibodies in all patients, which is unusual for vaccines," Yi told Wired. "We're talking about almost a 100 percent response rate. So far, we have seen an improvement in three out of three measurements of cognitive performance for patients with mild Alzheimer's disease."
The researchers also claim it can delay the onset of the disease by five years. While this would be a godsend for people with the disease and their families, according to Elle, it could also save Medicare and Medicaid more than $220 billion.
"You'd want to see larger numbers, but this looks like a beneficial treatment," James Brown, director of the Aston University Research Centre for Healthy Ageing, told Wired. "This looks like a silver bullet that can arrest or improve symptoms and, if it passes the next phase, it could be the best chance we've got."
"A word of caution is that it's a small study," says Drew Holzapfel, acting president of the nonprofit UsAgainstAlzheimer's, said according to Elle. "But the initial data is compelling."
The company is now working on its next clinical trial of the vaccine and while hopes are high, so is the pressure. The company has already invested $100 million developing its vaccine platform. According to Reese, the company's ultimate goal is to create a host of vaccines that will be administered to protect people from chronic illness.
"We have a 50-year vision -- to immuno-sculpt people against chronic illness and chronic aging with vaccines as prolific as vaccines for infectious diseases," he told Elle.
[Editor's Note: This article was originally published by Upworthy here and has been republished with permission.]
Turning Algae Into Environmentally Friendly Fuel Just Got Faster and Smarter
Algae in the Mediterranean sea.
Was your favorite beach closed this summer? Algae blooms are becoming increasingly the reason to blame and, as the climate heats up, scientists say we can expect more of the warm water-loving blue-green algae to grow.
"We have removed a significant development barrier to make algal biofuel production more efficient and smarter."
Oddly enough, the pesky growth could help fuel our carbon-friendly options.
This year, the University of Utah scientists discovered a faster way to turn algae into fuel. Algae is filled with lipids that we can feed our energy-hungry diesel engines. The problem is extracting the lipids, which usually requires more energy to transform than the actual energy we'd get – not achieving what scientists call "energy parity."
But now, the University of Utah team has discovered a new mix that is more efficient and much faster. We can now extract more power from algae with less waste materials after the fact. Paper co-author Dr. Leonard Pease says, "We have removed a significant development barrier to make algal biofuel production more efficient and smarter. Our method puts us much closer to creating biofuels energy parity than we were before."
Next Up
Algae has a lot going for it as an alternative fuel source. It grows fast and easily, absorbs carbon dioxide, does not compete with food crops for land, and could produce up to 60 times more oil than standard land-based energy crops, according to the U.S. Department of Energy. Yet the costs of algal biofuel production are still expensive for now.
According to Science Daily, only about five percent of total primary energy use in the United States came from algae and other biomass forms. By making the process more efficient, America and other nations could potentially begin relying on more plentiful resources – which, ironically, are more common now because of climate change.
Algae fuel efficiency is already a proven concept. A decade ago, Continental Airlines completed a 90-minute Boeing 737-800 flight with one engine split between biofuel and aircraft fuel. The biofuel was straight from algae. (Other flights were done based on nut fuel and other alternative sources.) The commercial airplane required no modification to the engine and the biofuel itself exceeded the standards of traditional jet fuel.
The problem, as noted at the time, is that biofuels derived from algae had yet to be proven as "commercially competitive."
The University of Utah's discovery could mean cheaper processing. At this point, it is less about if it works and more about if it is a practical alternative.
However, it's unclear how long it will take for algae to become more mainstream, if ever.
Open Questions
Higher efficiency and simpler transformations could mean lower prices and more business access. However, it's unclear how long it will take for algae to become more mainstream, if ever. The algae biofuel worked great for a relatively sophisticated Boeing 737 engine, but your family car, the cross-country delivery trucks and other less powerful machines may need to be modified – and that means the industry-at-large would have to revise their products in order to support the change.
Future-focused groups are already looking at how algae can fuel our space programs, especially if it is more renewable, safe and, potentially, cheaper than our traditional fuel choices. But first, it is worth waiting and seeing if corporations and, later, citizens are willing to take the plunge.