My Wife's Fight Against Cancer Inspired 38,000 People to Raise Millions for Research
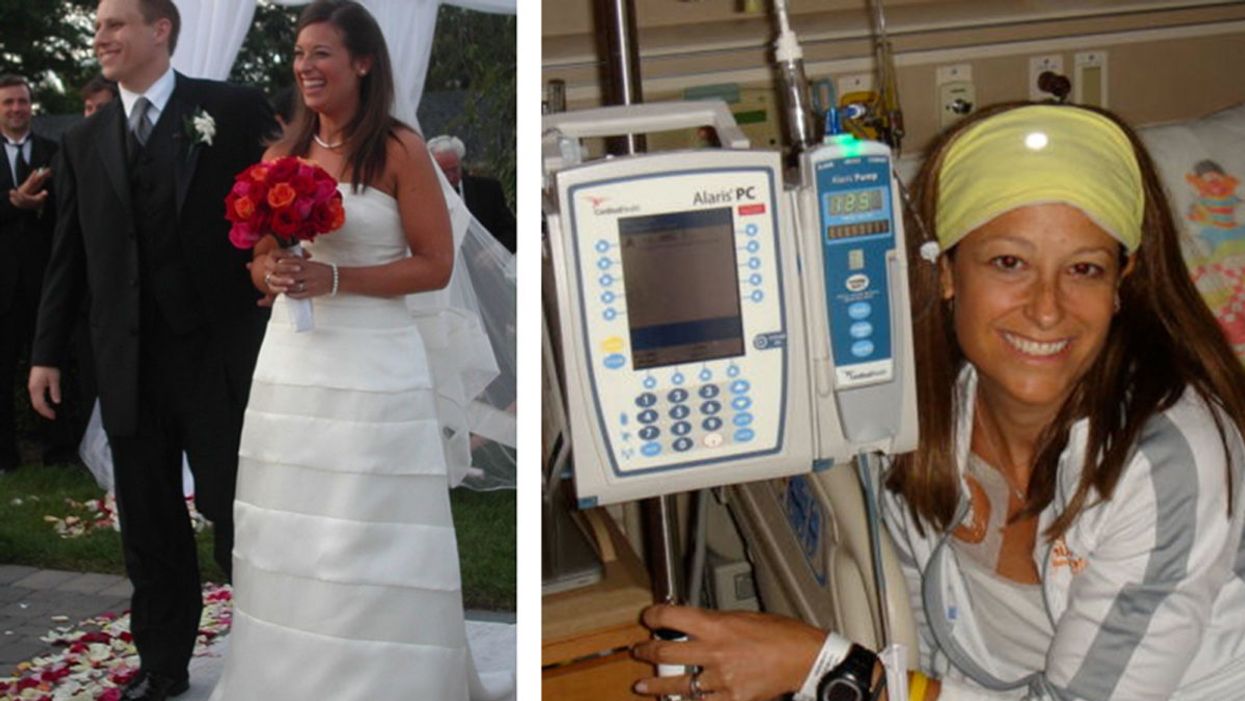
David and Jen on their wedding day, and Jen undergoing treatment for her rare sarcoma.
It was 15 years ago this month, but I'll never forget those words. When my wife Jen and I asked her oncologist about our plans to start a family, he calmly replied, "Well, I wouldn't do so unless Dave is prepared to be a single father."
About 50 percent of all people with cancer have a rare type, like the one Jen was fighting.
Time stood still. The danger crystalized — we were in a battle for my beautiful bride's life, and the odds were not in our favor.
We felt every emotion expected. Anger, sadness, confusion, frustration, and especially fear. But we made a very intentional choice to take that fear, put it to the side, and do everything we could to live our lives together to the fullest.
We focused first on Jen's health and learned everything we could about MFH Sarcoma. I was with her every step of the way — for hundreds of medical appointments, six intense surgeries, and twenty different types of chemotherapy. During such a challenging time, our choice to reject fear allowed us to live our best lives. Our careers blossomed, we enjoyed several international vacations, and Jen inspired thousands of fellow patients through her blog and speeches.
When we researched treatment options we learned that Jen was not alone. About 50 percent of all people with cancer have a rare type, like the one Jen was fighting. However, rare cancers don't get the funding they desperately need so effective treatment options are hard to find. The lack of funding felt unfair — and urgent. We didn't worry about everything that can go wrong when starting a new venture. Instead, we jumped in head first and convinced a small group of friends and family to ride stationary bikes with us to raise money for rare cancer research.

Jen Goodman Linn, riding a stationary bike for Cycle for Survival.
(Courtesy David Linn)
From those humble beginnings, Cycle for Survival grew steadily. After starting from scratch, Jen and I ran Cycle for Survival on our own for two years. We quickly realized that if we wanted to help as many people as possible, we needed the best partners. In 2009, we agreed that Memorial Sloan Kettering Cancer Center would take over the ownership of Cycle for Survival and Equinox officially became the Founding Partner. Flash forward to today, and Cycle for Survival has raised more than $220 million! I'm proud that 100% of every donation, yes every penny, goes directly into life-saving rare cancer research within six months of the annual indoor cycling events, which now take place in 17 cities nationwide.
While Cycle for Survival's trajectory was heading straight up, Jen's health struggle was devastatingly swinging up and down. With her incredible spirit and tenacity, Jen would beat the cancer through chemo and surgery, but then it would frustratingly come back again and again. After going into remission six times, it returned with such a vengeance in 2011 that even the world's leading doctors were forced to say, "I'm sorry, there's nothing more we can do."
Those were the most difficult words I've ever heard, by far. I hope no other family has to hear these crushing words.
When Jen died soon after, I didn't know what would happen to me, to my life, and to Cycle for Survival. I do remember making two very important choices at the time. First, I chose to get out of bed and put one foot in front of the other. It wasn't easy. Tears, pain, and grief would hit at any hour of the day or night. I did have a great support network of family and friends who kept me moving forward. One friend in particular changed the route of her morning runs so that I would join her and start getting back to exercising.
My second key choice was to stay involved with Cycle for Survival. At times, it was an excruciatingly difficult decision because I felt the depth of my loss each and every time I stepped into one of the events. However, it was also rewarding and energizing because I could see firsthand how many people it was helping, even though it was too late for Jen.
I began to travel across the country with the Cycle for Survival staff. My hope was to spread the word about rare cancers; along the way I met a lot of wonderful people who shared their stories with me. What I soon realized is that each of us faces obstacles in our lives. For me, it was losing the person who I wanted to spend my life with. For others, it might be challenges with their kids or in their professional lives. The common theme is that we don't have control over the fact that we have to face these challenges. But the biggest lesson I've learned is that we very much do have a choice in how we react.
I made the choice to do everything I can to help rare cancer patients and their families and it has been transformative and healing for me. The small group who rode in the first Cycle for Survival event has grown into a powerful movement of nearly 40,000 riders making a real difference. If Jen were diagnosed today, there are new treatments available– including genomic sequencing, targeted therapies, and immunotherapies – that could help her. Those weren't even options a short time ago. That's the result of funding research.

A recent Cycle for Survival event shows the passion and power of the community.
(Courtesy David Linn)
I also want to share one more choice I made. Remember that friend who changed the route of her morning runs so I could start exercising after Jen died? Well, over the years friendship grew into love, and we're now building a home together and can't wait to see what the future holds for us.
So with all that in mind I ask – when you face those inevitable challenges in your life, how will you choose to react? Remember that even in the midst of hopelessness, you can find choices. Those will be the decisions that define and guide you.
A new way to help kids with ADHD: Treat adult ADHD
Adults who have ADHD can benefit from treatment, and some researchers think their kids' ADHD symptoms may get better as a result.
When a child is diagnosed with attention deficit hyperactivity disorder (ADHD), it can often be a surprise to the parents that one of them has ADHD as well. They may have experienced some of the symptoms but never had the condition diagnosed.
Physicians, however, are usually less surprised because they know that ADHD is a very heritable disorder. According to a 2015 study, if a parent has ADHD, the child has up to a 57 percent chance of having it, and the child’s risk is 32 percent if their sibling has it.
“There have been 20 to 30 twin studies that show that the heritability of ADHD is about 70 percent,” meaning that both twins have it, says Stephen Faraone, distinguished professor and vice chair for research at SUNY Upstate Medical University. “It is as heritable as schizophrenia, bipolar disorder, autism or other psychiatric disorders that people tend to think are more biological than ADHD for some reason.”
More attention needed for adult ADHD
Brad McAlister, CMSE, executive director of the American Professional Society of ADHD & Related Disorders, or APSARD, explains that the consequences of untreated ADHD in adults are very well documented. The prevalence of ADHD in U.S. adults is 4.4 percent or about 11 million people.
Many adults go undiagnosed for decades or are misdiagnosed by providers. McAlister says that 75 percent are not receiving treatment. “The U.S. economic burden of adult ADHD is $105 to $194 billion annually,” he says. “The negative consequences on peoples’ lives include higher risks of dropping out of school, losing jobs, financial debt, divorce, fractured relationships, substance use disorders, and co-occurring depression/anxiety.”
One of the negative impacts of undiagnosed ADHD in adults is the effect that it can have on their children who have ADHD.
Adult ADHD is currently treated by a broad range of health care providers with different educational backgrounds and in different practice settings. In August, APSARD published the first U.S. guidelines for adult ADHD. “The creation of guidelines for ADHD in adults will allow all practitioners to benefit from the best evidence about diagnosing and treating the disorder,” McAlister says.
Faraone explains that the guidelines are intended to help practitioners understand the best practices for adults with ADHD, including screening and other ways of evaluating whether someone has it. He recently completed a study of what he calls the Metrics of Quality Care for adults with ADHD.
“We looked at a sizable group of primary care practices in the U.S., and we learned that although quality care for adults with ADHD has been gradually improving over the past decade, there are many areas where it is still far behind where it needs to be,” he says. “That’s consistent with other studies that show that in primary care for adults, ADHD is not treated nearly as well as it is treated in specialty and psychiatry care.”
How kids with ADHD are affected
One of the negative impacts of undiagnosed ADHD in adults is the effect that it can have on their children who have ADHD because their ability to care for that child’s special needs may be impaired.
“The treatments that are most effective in treating children with ADHD are medication and behavioral interventions as their reward bait, and at home, it’s the parent that administers them,” says Mark A. Stein, director of the ADHD and Related Disorders Program at Seattle Children’s Hospital. “Adults with ADHD have difficulties with time management and organization skills, so they will have a hard time making sure their child is ready for school, has breakfast, has their medications, etcetera.”
Even more challenging than getting a prescription, Stein adds, is finding a psychologist or therapist who is skilled in evaluating and working with children with ADHD and their parents. If left undiagnosed and untreated, adult ADHD may also interfere with getting a good evaluation for the child.
“If you have ADHD and your mind is wandering and you don’t have all of the forms from the school for your provider, and you’re focused on the bad day you’re having rather than giving a history of your child, all of that is going to delay getting an effective treatment for your child,” Stein says. “So that’s why it’s important to identify ADHD in parents.”
Promising research and training
After delays due to the pandemic, Stein and his colleague Andrea Chronis-Tuscano, professor and director of the Maryland ADHD Program at the University of Maryland, are now about two years into what they anticipate will be a six-year study that involves treating parents who have children with untreated ADHD symptoms. The goal is to see whether treating the parent first with medication and training, or just the training, helps the child’s symptoms due to improved parenting. They are also studying whether they can postpone the need for medication until children are older, when it’s more effective.
“Pediatricians are more aware of ADHD in parents because of our study,” Stein says. “They’re also more aware of the shortcomings in our healthcare delivery system in terms of how hard it is to find providers who are comfortable treating adult ADHD.”
“Besides depression, ADHD is the other disorder that parents have that really impacts kids significantly," Stein says. “With treatment, many people with ADHD do very well."
That said, he’s seen a significant improvement in the past decade with increased recognition of ADHD in adults. “It started with pediatricians recognizing that post-partum depression impacted the mother’s ability to care for her children and making it routine to screen for depression in parents of kids,” he says. “Besides depression, ADHD is the other disorder that parents have that really impacts kids significantly, so it’s important for them to be aware of characteristics of [ADHD in] parents and have resources they can give parents to help them.”
Stein emphasizes that even if someone displays symptoms of ADHD, that does not mean that they have it. They should seek a physician’s evaluation to confirm a diagnosis, which would enable them to get the medication and behavioral treatment they need.
The medication can take effect in parents within an hour. Meanwhile, when parents participate in the behavioral parent training courses, their kids with ADHD start showing significant improvement within about four to five weeks, according to Stein.
“With treatment, many people with ADHD do very well,” he says. “Especially if they get through formal schooling, find the right fit with their job, and if they make the right choices with their relationships, those three things can go a long way to make their ADHD fade into the background.”
Patients voice hope and relief as FDA gives third-ever drug approval for ALS
On Sept. 29, the FDA approved Relyvrio, a new drug for ALS, even though a study of 137 ALS patients did not result in “substantial evidence” that Relyvrio was effective.
At age 52, Glen Rouse suffered from arm weakness and a lot of muscle twitches. “I first thought something was wrong when I could not throw a 50-pound bag of dog food over the tailgate of my truck—something I use to do effortlessly,” said the 54-year-old resident of Anderson, California, about three hours north of San Francisco.
In August, Rouse retired as a forester for a private timber company, a job he had held for 31 years. The impetus: amyotrophic lateral sclerosis, or ALS, a progressive neuromuscular disease that is commonly known as Lou Gehrig’s disease, named after the New York Yankees’ first baseman who succumbed to it less than a month shy of his 38th birthday in 1941. ALS eventually robs an individual of the ability to talk, walk, chew, swallow and breathe.
Rouse is now dependent on ventilation through a nasal mask and uses a powerchair to get around. “I can no longer walk or use my arms very well,” he said. “I can still move my wrists and fingers. I can also transfer from my chair to the toilet if I have two of my friends help me.”
It’s “shocking” that modern medicine has very little to offer to people with this devastating condition, Rouse said. But there is hope on the horizon. Yesterday, the U.S. Food and Drug Administration approved Relyvrio, a drug made up of two parts, sodium phenylbutyrate and taurursodiol, to treat patients with ALS.
“This approval provides another important treatment option for ALS, a life-threatening disease that currently has no cure,” said Billy Dunn, director of the Office of Neuroscience in the FDA’s Center for Drug Evaluation and Research, in a statement. “The FDA remains committed to facilitating the development of additional ALS treatments.”
Until this point, the FDA had approved only two other medications—Riluzole (rilutek) in 1995 and Radicava (edaravone) in 2017—to extend life in patients with ALS, which typically kills within two to five years after diagnosis. That’s why earlier this week, Rouse was optimistic about the FDA’s likely approval of a controversial new drug for ALS.
When Relyvrio is taken in addition to Riluzole, it appears to slow functional decline by an additional 25 percent and extend life by another 6 to 10 months, said Richard Bedlak, director of the Duke ALS Clinic. “It is not a cure, but it is definitely a step forward.”
“The whole ALS community is extremely excited about it,” he said the day before Relyvrio’s expected approval. “We are very hopeful. We’re on pins and needles.”
A study of 137 ALS patients did not result in “substantial evidence” that Relyvrio was effective, the agency’s Peripheral and Central Nervous System Drugs Advisory Committee concluded in March. However, after some persuasion from FDA officials, patients and their families, the committee met again and decided to recommend approving the drug.
In January 2019, following an ALS diagnosis at age 58 in October the previous year, Jeff Sarnacki, of Chester, Maryland, was accepted into a trial for Relyvrio. “Because of the trial, we did experience hope and a greater sense of help than had we not had that opportunity,” said Juliet Taylor, his wife and caregiver. They both believed the drug “worked for him in giving him more time.”
In June 2019, Sarnacki chose an open-label extension, offered to patients by drug researchers after a study ends, and took the active drug until he died peacefully at home under hospice care in May 2020, five days after his 60th birthday. A retired agent with the federal Bureau of Alcohol, Tobacco, Firearms and Explosives who later worked as a security consultant, Sarnacki lived about 19 months after diagnosis, which is shorter than the typical prognosis.
His symptoms began with leg cramps in fall 2017 and foot drop in early 2018. A feeding tube was placed in 2019, as it became necessary early in his illness, Taylor said. He also took Radicava and Riluzole, the two previously approved drugs, for his ALS. “We were both incredulous that, so many years after Lou Gehrig’s own diagnosis, there were so few treatments available,” she said.
The dearth of successful treatments for ALS is “certainly not for lack of trying,” said Karen Raley Steffens, a registered nurse and ALS support services coordinator at the Les Turner ALS Foundation in Skokie, Ill. “There are thousands of researchers and scientists all over the world working tirelessly to try to develop treatments for ALS.”
Unfortunately, she added, research takes time and exorbitant amounts of funding, while bureaucratic challenges persist. The rare disease also manifests and progresses in many different ways, so many treatments are needed.
As of 2017, the Centers for Disease Control and Prevention estimated that more than 31,000 people in the U.S. live with ALS, and an average of 5,000 people are newly diagnosed every year. It is slightly more common in men than women. Most people are diagnosed between the ages of 55 and 75.
Most cases of ALS are sporadic, meaning that doctors don’t know the cause. There is about a one-year interval between symptom onset and an ALS diagnosis for most patients, so many motor neurons are lost by the time individuals can enroll in a clinical trial, said Richard Bedlack, professor of neurology and director of the Duke ALS Clinic in Durham, North Carolina.
Bedlack found the new drug, Relyvrio, to be “very promising,” which is why he testified to the FDA in favor of approval. (He’s a consultant and disease state speaker for multiple companies including Amylyx, manufacturer of Relyvrio.)
The “drug has different mechanisms of action than the currently approved treatments,” Bedlack said. He added that, when Relyvrio is taken in addition to Riluzole, it appears to slow functional decline by an additional 25 percent and extend life by another 6 to 10 months. “It is not a cure, but it is definitely a step forward.”
T. Scott Diesing, a neurohospitalist and director of general neurology at the University of Nebraska Medical Center in Omaha, said he hopes the drug is “as good as people anticipated it should be, because there are not too many options for these patients.”
"FDA went out on a limb in approving Relyvrio based on limited results from a small trial while a larger study remains in progress," said Florian P. Thomas, co-director of the ALS Center at Hackensack University Medical Center and Hackensack Meridian School of Medicine in New Jersey. "While it is definitely promising, clearly, the last word on this drug has not been spoken."
So far, Rouse's voice is holding up, but he knows the day will come when ALS will steal that and much more from him.
ALS is 100 percent fatal, with some patients dying as soon as a year after diagnosis. A few have lasted as long as 15 years, but those are the exceptions, Diesing said.
“If this drug can provide even months of additional life, or would maintain quality of life, that’s a big deal,” he noted, adding that “the patients are saying, ‘I know it’s not proven conclusively, but what do we have to lose?’ So, they would like to try it while additional studies are ongoing.” The drug has already been conditionally approved in Canada.
As his disease progresses, Rouse hopes to get a speech-to-text voice-generating computer that he can control with his eyes. So far, his voice is holding up, but he knows the day will come when ALS will steal that and much more from him. He works at I AM ALS, a patient-led community, and six of his friends have already died of the disease.
“Every time I lose a friend to ALS, I grieve and am sad but I resolve myself to keep working harder for them, myself and others,” Rouse said. “People living with ALS find great purpose in life advocating and trying to make a difference.”

