Tiny, Injectable Robots Could Be the Future of Brain Treatments
A movie still from the 1966 film "Fantastic Voyage"
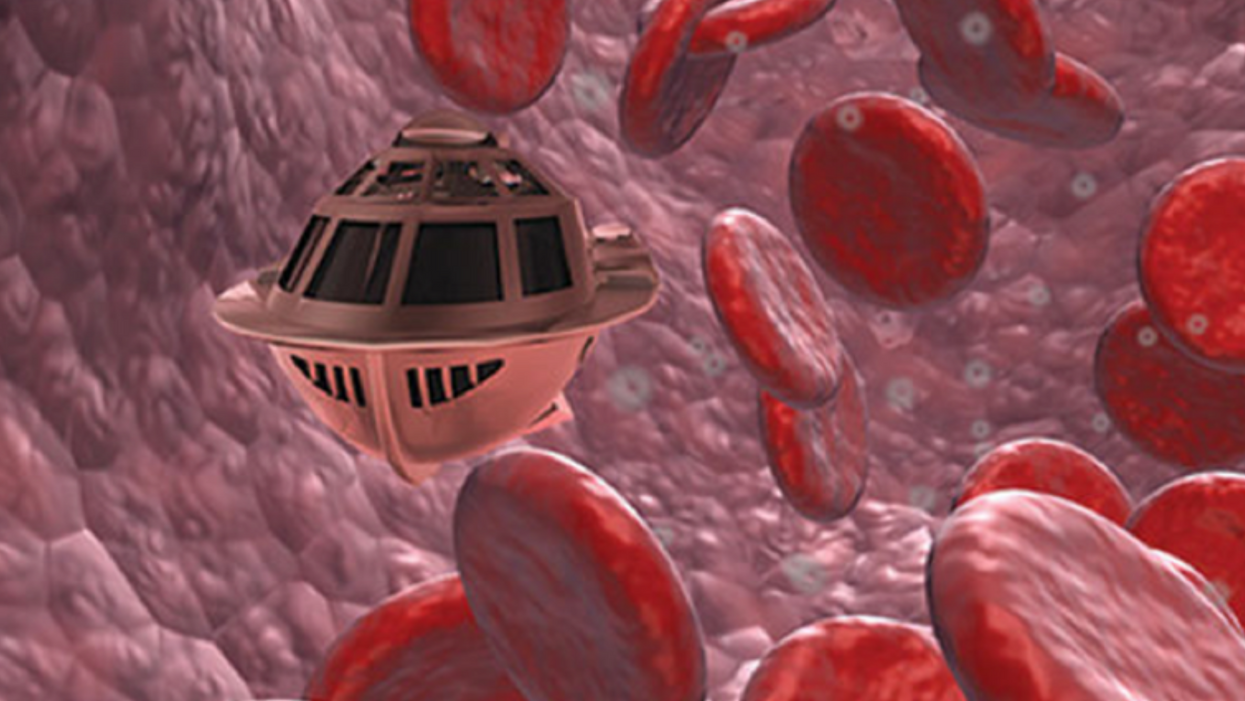
In the 1966 movie "Fantastic Voyage," actress Raquel Welch and her submarine were shrunk to the size of a cell in order to eliminate a blood clot in a scientist's brain. Now, 55 years later, the scenario is becoming closer to reality.
California-based startup Bionaut Labs has developed a nanobot about the size of a grain of rice that's designed to transport medication to the exact location in the body where it's needed. If you think about it, the conventional way to deliver medicine makes little sense: A painkiller affects the entire body instead of just the arm that's hurting, and chemotherapy is flushed through all the veins instead of precisely targeting the tumor.
"Chemotherapy is delivered systemically," Bionaut-founder and CEO Michael Shpigelmacher says. "Often only a small percentage arrives at the location where it is actually needed."
But what if it was possible to send a tiny robot through the body to attack a tumor or deliver a drug at exactly the right location?
Several startups and academic institutes worldwide are working to develop such a solution but Bionaut Labs seems the furthest along in advancing its invention. "You can think of the Bionaut as a tiny screw that moves through the veins as if steered by an invisible screwdriver until it arrives at the tumor," Shpigelmacher explains. Via Zoom, he shares the screen of an X-ray machine in his Culver City lab to demonstrate how the half-transparent, yellowish device winds its way along the spine in the body. The nanobot contains a tiny but powerful magnet. The "invisible screwdriver" is an external magnetic field that rotates that magnet inside the device and gets it to move and change directions.
The current model has a diameter of less than a millimeter. Shpigelmacher's engineers could build the miniature vehicle even smaller but the current size has the advantage of being big enough to see with bare eyes. It can also deliver more medicine than a tinier version. In the Zoom demonstration, the micorobot is injected into the spine, not unlike an epidural, and pulled along the spine through an outside magnet until the Bionaut reaches the brainstem. Depending which organ it needs to reach, it could be inserted elsewhere, for instance through a catheter.
"The hope is that we can develop a vehicle to transport medication deep into the body," says Max Planck scientist Tian Qiu.
Imagine moving a screw through a steak with a magnet — that's essentially how the device works. But of course, the Bionaut is considerably different from an ordinary screw: "At the right location, we give a magnetic signal, and it unloads its medicine package," Shpigelmacher says.
To start, Bionaut Labs wants to use its device to treat Parkinson's disease and brain stem gliomas, a type of cancer that largely affects children and teenagers. About 300 to 400 young people a year are diagnosed with this type of tumor. Radiation and brain surgery risk damaging sensitive brain tissue, and chemotherapy often doesn't work. Most children with these tumors live less than 18 months. A nanobot delivering targeted chemotherapy could be a gamechanger. "These patients really don't have any other hope," Shpigelmacher says.
Of course, the main challenge of the developing such a device is guaranteeing that it's safe. Because tissue is so sensitive, any mistake could risk disastrous results. In recent years, Bionaut has tested its technology in dozens of healthy sheep and pigs with no major adverse effects. Sheep make a good stand-in for humans because their brains and spines are similar to ours.

The Bionaut device is about the size of a grain of rice.
Bionaut Labs
"As the Bionaut moves through brain tissue, it creates a transient track that heals within a few weeks," Shpigelmacher says. The company is hoping to be the first to test a nanobot in humans. In December 2022, it announced that a recent round of funding drew $43.2 million, for a total of 63.2 million, enabling more research and, if all goes smoothly, human clinical trials by early next year.
Once the technique has been perfected, further applications could include addressing other kinds of brain disorders that are considered incurable now, such as Alzheimer's or Huntington's disease. "Microrobots could serve as a bridgehead, opening the gateway to the brain and facilitating precise access of deep brain structure – either to deliver medication, take cell samples or stimulate specific brain regions," Shpigelmacher says.
Robot-assisted hybrid surgery with artificial intelligence is already used in state-of-the-art surgery centers, and many medical experts believe that nanorobotics will be the instrument of the future. In 2016, three scientists were awarded the Nobel Prize in Chemistry for their development of "the world's smallest machines," nano "elevators" and minuscule motors. Since then, the scientific experiments have progressed to the point where applicable devices are moving closer to actually being implemented.
Bionaut's technology was initially developed by a research team lead by Peer Fischer, head of the independent Micro Nano and Molecular Systems Lab at the Max Planck Institute for Intelligent Systems in Stuttgart, Germany. Fischer is considered a pioneer in the research of nano systems, which he began at Harvard University more than a decade ago. He and his team are advising Bionaut Labs and have licensed their technology to the company.
"The hope is that we can develop a vehicle to transport medication deep into the body," says Max Planck scientist Tian Qiu, who leads the cooperation with Bionaut Labs. He agrees with Shpigelmacher that the Bionaut's size is perfect for transporting medication loads and is researching potential applications for even smaller nanorobots, especially in the eye, where the tissue is extremely sensitive. "Nanorobots can sneak through very fine tissue without causing damage."
In "Fantastic Voyage," Raquel Welch's adventures inside the body of a dissident scientist let her swim through his veins into his brain, but her shrunken miniature submarine is attacked by antibodies; she has to flee through the nerves into the scientist's eye where she escapes into freedom on a tear drop. In reality, the exit in the lab is much more mundane. The Bionaut simply leaves the body through the same port where it entered. But apart from the dramatization, the "Fantastic Voyage" was almost prophetic, or, as Shpigelmacher says, "Science fiction becomes science reality."
This article was first published by Leaps.org on April 12, 2021.
Dec. 17th Event: The Latest on Omicron, Boosters, and Immunity
The Omicron variant poses new uncertainty for the vaccines, which four leading experts will address during our virtual event on December 17th, 2021.
This virtual event will convene leading scientific and medical experts to discuss the most pressing questions around the new Omicron variant, including what we know so far about its ability to evade COVID-19 vaccines, the role of boosters in eliciting heightened immunity, and the science behind variants and vaccines. A public Q&A will follow the expert discussion.
EVENT INFORMATION:
Date: Friday Dec 17, 2021
2:00pm - 3:30pm EST

Dr. Céline Gounder, MD, ScM, is the CEO/President/Founder of Just Human Productions, a non-profit multimedia organization. She is also the host and producer of American Diagnosis, a podcast on health and social justice, and Epidemic, a podcast about infectious disease epidemics and pandemics. She served on the Biden-Harris Transition COVID-19 Advisory Board.
 Dr. Theodora Hatziioannou, Ph.D., is a Research Associate Professor in the Laboratory of Retrovirology at The Rockefeller University. Her research includes identifying plasma samples from recovered COVID-19 patients that contain antibodies capable of neutralizing the SARS-CoV-2 coronavirus.
Dr. Theodora Hatziioannou, Ph.D., is a Research Associate Professor in the Laboratory of Retrovirology at The Rockefeller University. Her research includes identifying plasma samples from recovered COVID-19 patients that contain antibodies capable of neutralizing the SARS-CoV-2 coronavirus.

Dr. Onyema Ogbuagu, MBBCh, is an Associate Professor at Yale School of Medicine and an infectious disease specialist who treats COVID-19 patients and leads Yale’s clinical studies around COVID-19. He ran Yale’s trial of the Pfizer/BioNTech vaccine.

Dr. Eric Topol, M.D., is a cardiologist, scientist, professor of molecular medicine, and the director and founder of Scripps Research Translational Institute. He has led clinical trials in over 40 countries with over 200,000 patients and pioneered the development of many routinely used medications.
This event is the fourth of a four-part series co-hosted by Leaps.org, the Aspen Institute Science & Society Program, and the Sabin–Aspen Vaccine Science & Policy Group, with generous support from the Gordon and Betty Moore Foundation and the Howard Hughes Medical Institute.

Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
7 Things to Know about the U.S.’s Capability to Detect Omicron
A visualization of the original coronavirus causing COVID-19, which has now been detected across the globe in a highly mutated form called Omicron.
If the new variant Omicron isn’t here already – which many experts suspect that it is – it will be soon. While we wait for scientists to conduct the necessary research to characterize its transmissibility, potential fitness at immune evasion, and disease severity, we wanted to give Leaps.org readers a window into how the U.S. is positioned to detect the variant. So we spoke to Kelly Wroblewski, director of infectious diseases at the Association of Public Health Laboratories, a membership organization that represents state and local government health labs in the United States. Here are seven insights she shared.
1) If you test positive for COVID-19 with a standard PCR test, the diagnostic report will not tell you which variant you have. There are no diagnostic tests available for your doctor to order to identify variants. To find out the variant, the specimen must be sent to a commercial, clinical, academic, or public health laboratory for genetic sequencing.
2) Today, the U.S. sequences about 5 to 10 percent of all diagnostic specimens that test positive for SARS-CoV-2 in order to determine which variants are circulating and where. Last week nationally, for example, labs sequenced about 80,000 samples. This represents a massive increase from last year at this time, when labs were only sequencing about 8,000 specimens per week. Currently, 99.5 percent of circulating SARS-CoV-2 virus in the U.S. is the Delta variant.
3) The U.S. is “very well prepared” to detect Omicron, Wroblewski says, “particularly compared to where we were when the Alpha variant, or B117 first emerged.” Of the hunt for Omicron, she adds, “it’s very reminiscent of that time, except we are doing so much more sequencing and we have so much better coverage with our sequencing geographically, and we're doing it in a much more timely way. We have the ability to find emerging variants that are circulating in 0.01 percent of the population.”
4) Deciding which specimens to sample is not totally random. Samples that have more virus are likely to lead to better sequencing results. Labs also look to have a diverse set of representative samples, meaning across geographic regions and across gender, race, ethnicity, and age groups. Clinical diversity is also important, such as including pregnant women, severe in-patient cases, mild cases, etc.
5) Sequencing more is not necessarily better to find Omicron faster. “We will increase the number of sequences to a certain extent,” Wroblewski says. “Where we exhibit some caution is doing that indiscriminately isn’t the most effective use of time and resources. The important thing is to try to find Omicron, and if you increase your testing capacity too much, right now, it's still predominantly Delta in the U.S. by a long shot. So you’re mostly going to sequence Delta and you run the risk of delaying your discovery of Omicron, if you focus solely on increasing sequencing.”
So besides just ramping up the sheer numbers of sequencing, diagnostic labs across the country are now advised to preferentially use a certain PCR test made by Thermo Fisher that can help hasten the detection of Omicron. It turns out that Omicron’s specific mutations in the Spike protein mean that the Spike is not picked up on this PCR test, which yields a type of result called an S-gene target failure. Yet the test will still accurately pick up a COVID-19 diagnosis, because it detects two other gene targets on Omicron that are not mutated. “That S-gene target failure gives you a good indication that you may have Omicron. It’s a good early screen.”
Labs will then still need to sequence the whole genome to confirm it matches the Omicron sequence. “So right now, the new recommendation is to use [the Thermo Fisher test] as much as possible to give us a better chance of detecting Omicron more quickly.”
6) This Thermo Fisher test is “fairly widely used” in the U.S. already, so many labs are already well positioned to make the shift. “In early to mid 2020,” Wroblewski explains, “when the supply chain issue for testing was acute, many public health labs implemented five, six, seven, eight different tests, just so they could get enough supplies to do all the testing. Now that we're in a much better place supply-chain wise, it's very difficult and time consuming and cumbersome to maintain all those different test methods all the time, and many, many labs scaled back to only one or two. And so this [new recommendation] would just be shifting to two for some labs that will be shifting to them.”
7) Once Omicron is found here, labs will be focused on finding as many cases as possible, and the CDC will be conducting a variety of studies to determine the impact of the variant on diagnostics, therapeutics, and vaccines. Epidemiologists at the local, state, and federal level will analyze which populations it is spreading in, as well as the severity of the disease it causes. They will work to sort out different impacts on vaccinated vs. unvaccinated populations. The ultimate goal, Wroblewski concludes, is to “use all of that information to make better public health decisions and inform the public about what’s going on.”
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.