New Options Are Emerging in the Search for Better Birth Control
A decade ago, Elizabeth Summers' options for birth control suddenly narrowed. Doctors diagnosed her with Factor V Leiden, a rare genetic disorder, after discovering blood clots in her lungs. The condition increases the risk of clotting, so physicians told Summers to stay away from the pill and other hormone-laden contraceptives. "Modern medicine has generally failed to provide me with an effective and convenient option," she says.
But new birth control options are emerging for women like Summers. These alternatives promise to provide more choices to women who can't ingest hormones or don't want to suffer their unpleasant side effects.
These new products have their own pros and cons. Still, doctors are welcoming new contraceptives following a long drought in innovation. "It's been a long time since we've had something new in the world of contraception," says Heather Irobunda, an obstetrician and gynecologist at NYC Health and Hospitals.
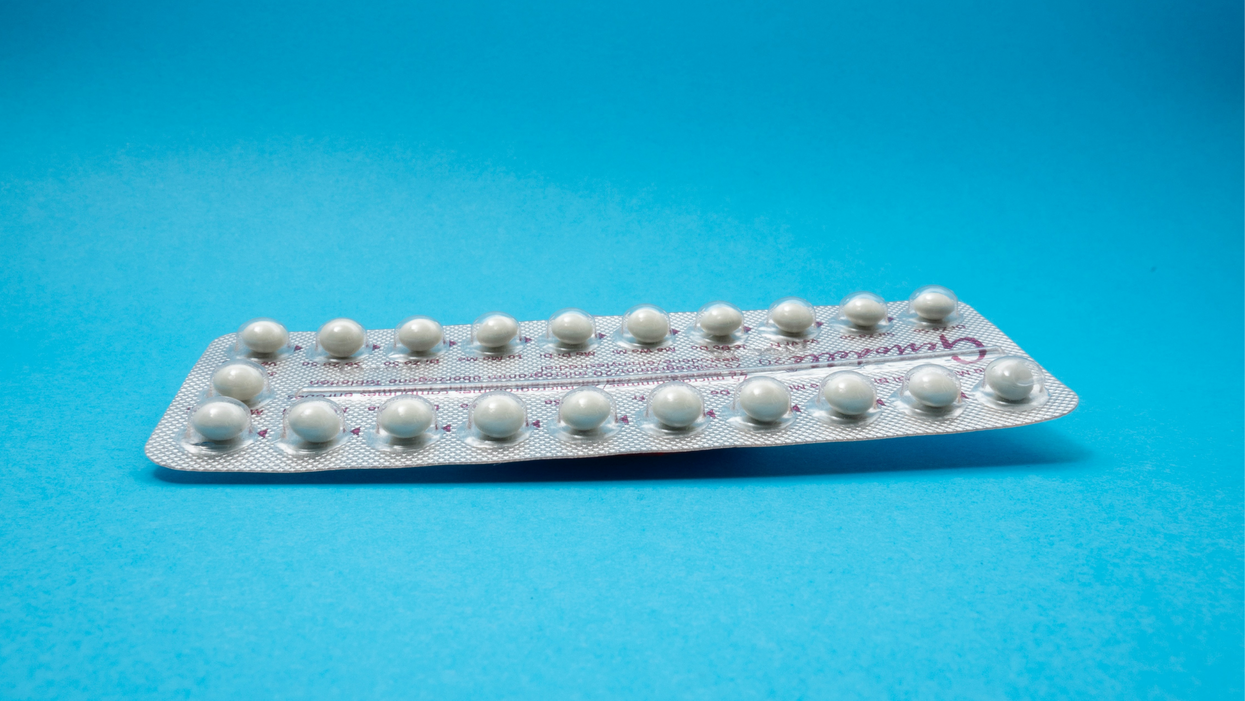
On social media, Irobunda often fields questions about one of these new options, a lubricating gel called Phexxi. San Diego-based Evofem, the company behind Phexxi, has been advertising the product on Hulu and Instagram after the gel was approved by the Food and Drug Administration in May 2020. The company's trendy ads target women who feel like condoms diminish the mood, but who also don't want to mess with an IUD or hormones.
Here's how it works: Phexxi is inserted via a tampon-like device up to an hour before sex. The gel regulates vaginal pH — essentially, the acidity levels — in a range that's inhospitable to sperm. It sounds a lot like spermicide, which is also placed in the vagina prior to sex to prevent pregnancy. But spermicide can damage the vagina's cell walls, which can increase the risk of contracting sexually transmitted diseases.
"Not only is innovation needed, but women want a non-hormonal option."
Phexxi isn't without side effects either. The most common one is vaginal burning, according to a late-stage trial. It's also possible to develop a urinary tract infection while using the product. That same study found that during typical use, Phexxi is about 86 percent effective at preventing pregnancy. The efficacy rate is comparable to condoms but lower than birth control pills (91 percent) and significantly lower than an IUD (99 percent).
Phexxi – which comes in a pack of 12 – represents a tiny but growing part of the birth control market. Pharmacies dispensed more than 14,800 packs from April through June this year, a 65 percent increase over the previous quarter, according to data from Evofem.
"We've been able to demonstrate that not only is innovation needed, but women want a non-hormonal option," says Saundra Pelletier, Evofem's CEO.
Beyond contraception, the company is carrying out late-stage tests to gauge Phexxi's effectiveness at preventing the sexually transmitted infections chlamydia and gonorrhea.

Phexxi is inserted via a tampon-like device up to an hour before sex.
Phexxi
A New Pill
The first birth control pill arrived in 1960, combining the hormones estrogen and progestin to stop sperm from joining with an egg, giving women control over their fertility. Subsequent formulations sought to ease side effects, by way of lower amounts of estrogen. But some women still experience headaches and nausea – or more serious complications like blood clots. On social media, women recently noted that birth control pills are much more likely to cause blood clots than Johnson & Johnson's COVID-19 vaccine that was briefly paused to evaluate the risk of clots in women under age 50. What will it take, they wondered, for safer birth control?
Mithra Pharmaceuticals of Belgium sought to create a gentler pill. In April, the FDA approved Mithra's Nextstellis, which includes a naturally occurring estrogen, the first new estrogen in the U.S. in 50 years. Nextstellis selectively acts on tissues lining the uterus, while other birth control pills have a broader target.
A Phase 3 trial showed a 98 percent efficacy rate. Andrew London, an obstetrician and gynecologist, who practices at several Maryland hospitals, says the results are in line with some other birth control pills. But, he added, early studies indicate that Nextstellis has a lower risk of blood clotting, along with other potential benefits, which additional clinical testing must confirm.
"It's not going to be worse than any other pill. We're hoping it's going to be significantly better," says London.
The estrogen in Nexstellis, called estetrol, was skipped over by the pharmaceutical industry after its discovery in the 1960s. Estetrol circulates between the mother and fetus during pregnancy. Decades later, researchers took a new look, after figuring out how to synthesize estetrol in a lab, as well as produce estetrol from plants.
"That allowed us to really start to investigate the properties and do all this stuff you have to do for any new drug," says Michele Gordon, vice president of marketing in women's health at Mayne Pharma, which licensed Nextstellis.
Bonnie Douglas, who followed the development of Nextstellis as part of a search for better birth control, recently switched to the product. "So far, it's much more tolerable," says Douglas. Previously, the Midwesterner was so desperate to find a contraceptive with fewer side effects that she turned to an online pharmacy to obtain a different birth control pill that had been approved in Canada but not in the U.S.
Contraceptive Access
Even if a contraceptive lands FDA approval, access poses a barrier. Getting insurers to cover new contraceptives can be difficult. For the uninsured, state and federal programs can help, and companies should keep prices in a reasonable range, while offering assistance programs. So says Kelly Blanchard, president of the nonprofit Ibis Reproductive Health. "For innovation to have impact, you want to reach as many folks as possible," she says.
In addition, companies developing new contraceptives have struggled to attract venture capital. That's changing, though.
In 2015, Sabrina Johnson founded DARÉ Bioscience around the idea of women's health. She estimated the company would be fully funded in six months, based on her track record in biotech and the demand for novel products.
But it's been difficult to get male investors interested in backing new contraceptives. It took Johnson two and a half years to raise the needed funds, via a reverse merger that took the company public. "There was so much education that was necessary," Johnson says, adding: "The landscape has changed considerably."
Johnson says she would like to think DARÉ had something to do with the shift, along with companies like Organon, a spinout of pharma company Merck that's focused on reproductive health. In surveying the fertility landscape, DARÉ saw limited non-hormonal options. On-demand options – like condoms – can detract from the moment. Copper IUDs must be inserted by a doctor and removed if a woman wants to return to fertility, and this method can have onerous side effects.
So, DARÉ created Ovaprene, a hormone-free device that's designed to be inserted into the vagina monthly by the user. The mesh product acts as a barrier, while releasing a chemical that immobilizes sperm. In an early study, the company reported that Ovaprene prevented almost all sperm from entering the cervical canal. The results, DARÉ believes, indicate high efficacy.
A late-stage study, slated to kick off next year, will be the true judge. Should Ovaprene eventually win regulatory approval, drug giant Bayer will handle commercializing the device.
Other new forms of birth control in development are further out, and that's assuming they perform well in clinical trials. Among them: a once-a-month birth control pill, along with a male version of the birth control pill. The latter is often brought up among women who say it's high time that men take a more proactive role in birth control.
For Summers, her search for a safe and convenient birth control continues. She tried Phexxi, which caused irritation. Still, she's excited that a non-hormonal option now exists. "I'm sure it will work for others," she says.
World’s First “Augmented Reality” Contact Lens Aims to Revolutionize Much More Than Medicine
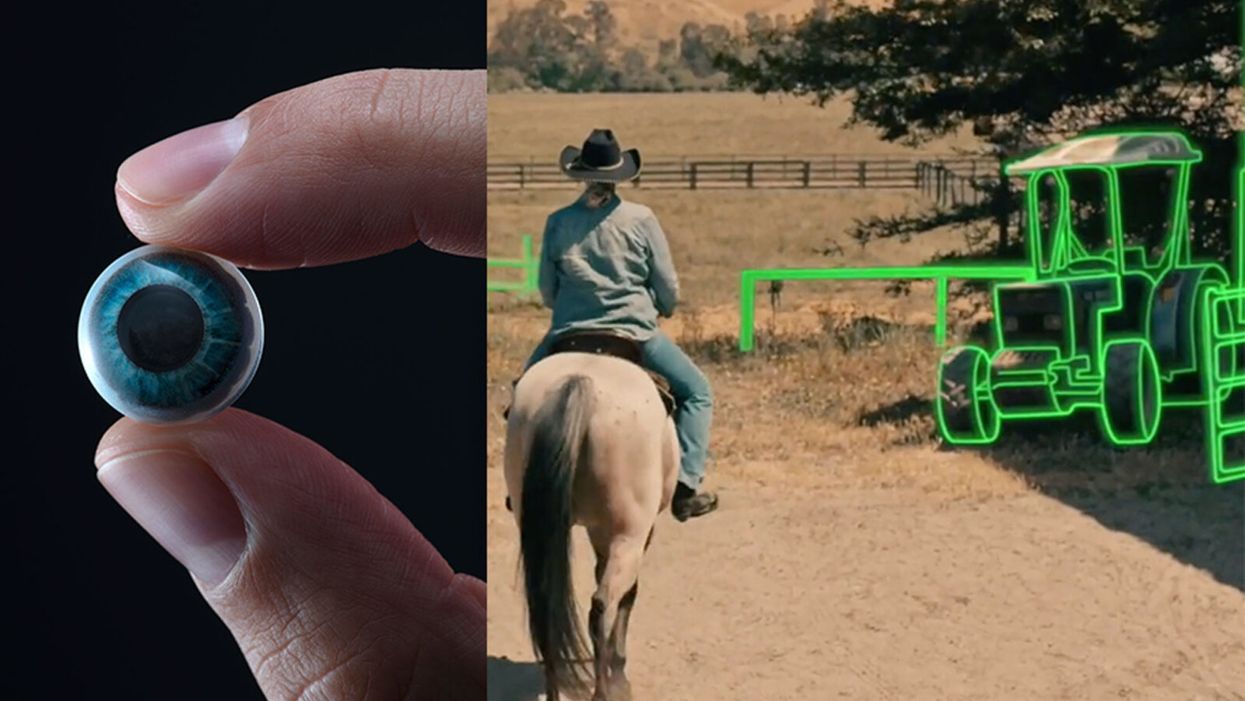
On the left, a picture of the Mojo lens smart contact; and a simulated image of a woman with low vision who is wearing the contact to highlight objects in her field of vision.
Imagine a world without screens. Instead of endlessly staring at your computer or craning your neck down to scroll through social media feeds and emails, information simply appears in front of your eyes when you need it and disappears when you don't.
"The vision is super clear...I was reading the poem with my eyes closed."
No more rude interruptions during dinner, no more bumping into people on the street while trying to follow GPS directions — just the information you want, when you need it, projected directly onto your visual field.
While this screenless future sounds like science fiction, it may soon be a reality thanks to the new Silicon Valley startup Mojo Vision, creator of the world's first smart contact lens. With a 14,000 pixel-per-inch display with eye-tracking, image stabilization, and a custom wireless radio, the Mojo smart lens bills itself the "smallest and densest dynamic display ever made." Unlike current augmented reality wearables such as Google Glass or ThirdEye, which project images onto a glass screen, the Mojo smart lens can project images directly onto the retina.
A current prototype displayed at the Consumer Electronics Show earlier this year in Las Vegas includes a tiny screen positioned right above the most sensitive area of the pupil. "[The Mojo lens] is a contact lens that essentially has wireless power and data transmission for a small micro LED projector that is placed over the center of the eye," explains David Hobbs, Director of Product Management at Mojo Vision. "[It] displays critical heads-up information when you need it and fades into the background when you're ready to continue on with your day."
Eventually, Mojo Visions' technology could replace our beloved smart devices but the first generation of the Mojo smart lens will be used to help the 2.2 billion people globally who suffer from vision impairment.
"If you think of the eye as a camera [for the visually impaired], the sensors are not working properly," explains Dr. Ashley Tuan, Vice President of Medical Devices at Mojo Vision and fellow of the American Academy of Optometry. "For this population, our lens can process the image so the contrast can be enhanced, we can make the image larger, magnify it so that low-vision people can see it or we can make it smaller so they can check their environment." In January of this year, the FDA granted Breakthrough Device Designation to Mojo, allowing them to have early and frequent discussions with the FDA about technical, safety and efficacy topics before clinical trials can be done and certification granted.
For now, Dr. Tuan is one of the few people who has actually worn the Mojo lens. "I put the contact lens on my eye. It was very comfortable like any contact lenses I've worn before," she describes. "The vision is super clear and then when I put on the accessories, suddenly I see Yoda in front of me and I see my vital signs. And then I have my colleague that prepared a beautiful poem that I loved when I was young [and] I was reading the poem with my eyes closed."
At the moment, there are several electronic glasses on the market like Acesight and Nueyes Pro that provide similar solutions for those suffering from visual impairment, but they are large, cumbersome, and highly visible. Mojo lens would be a discreet, more comfortable alternative that offers users more freedom of movement and independence.
"In the case of augmented-reality contact lenses, there could be an opportunity to improve the lives of people with low vision," says Dr. Thomas Steinemann, spokesperson for the American Academy of Ophthalmology and professor of ophthalmology at MetroHealth Medical Center in Cleveland. "There are existing tools for people currently living with low vision—such as digital apps, magnifiers, etc.— but something wearable could provide more flexibility and significantly more aid in day-to-day tasks."
As one of the first examples of "invisible computing," the potential applications of Mojo lens in the medical field are endless.
According to Dr. Tuan, the visually impaired often suffer from depression due to their lack of mobility and 70 percent of them are underemployed. "We hope that they can use this device to gain their mobility so they can get that social aspect back in their lives and then, eventually, employment," she explains. "That is our first and most important goal."
But helping those with low visual capabilities is only Mojo lens' first possible medical application; augmented reality is already being used in medicine and is poised to revolutionize the field in the coming decades. For example, Accuvein, a device that uses lasers to provide real-time images of veins, is widely used by nurses and doctors to help with the insertion of needles for IVs and blood tests.
According to the National Center for Biotechnology Information, augmentation of reality has been used in surgery for many years with surgeons using devices such as Google Glass to overlay critical information about their patients into their visual field. Using software like the Holographic Navigation Platform by Scopsis, surgeons can see a mixed-reality overlay that can "show you complicated tumor boundaries, assist with implant placements and guide you along anatomical pathways," its developers say.
However, according to Dr. Tuan, augmented reality headsets have drawbacks in the surgical setting. "The advantage of [Mojo lens] is you don't need to worry about sweating or that the headset or glasses will slide down to your nose," she explains "Also, our lens is designed so that it will understand your intent, so when you don't want the image overlay it will disappear, it will not block your visual field, and when you need it, it will come back at the right time."
As one of the first examples of "invisible computing," the potential applications of Mojo lens in the medical field are endless. Possibilities include live translation of sign language for deaf people; helping those with autism to read emotions; and improving doctors' bedside manner by allowing them to fully engage with patients without relying on a computer.
"[By] monitoring those blood vessels we can [track] chronic disease progression: high blood pressure, diabetes, and Alzheimer's."
Furthermore, the lens could be used to monitor health issues. "We have image sensors in the lens right now that point to the world but we can have a camera pointing inside of your eye to your retina," says Dr. Tuan, "[By] monitoring those blood vessels we can [track] chronic disease progression: high blood pressure, diabetes, and Alzheimer's."
For the moment, the future medical applications of the Mojo lens are still theoretical, but the team is confident they can eventually become a reality after going through the proper regulatory review. The company is still in the process of design, prototype and testing of the lens, so they don't know exactly when it will be available for use, but they anticipate shipping the first available products in the next couple of years. Once it does go to market, it will be available by prescription only for those with visual impairments, but the team's goal is to bring it to broader consumer markets pending regulatory clearance.
"We see that right now there's a unique opportunity here for Mojo lens and invisible computing to help to shape what the next decade of technology development looks like," explains David Hobbs. "We can use [the Mojo lens] to better serve us as opposed to us serving technology better."
Schmidt Ocean Institute co-founder Wendy Schmidt is backed by 32 screens in research vessel Falkor's control room where most of the science takes place on the ship, from mapping to live streaming of underwater robotic dives.
WENDY SCHMIDT is a philanthropist and investor who has spent more than a dozen years creating innovative non-profit organizations to solve pressing global environmental and human rights issues. Recognizing the human dependence on sustaining and protecting our planet and its people, Wendy has built organizations that work to educate and advance an understanding of the critical interconnectivity between the land and the sea. Through a combination of grants and investments, Wendy's philanthropic work supports research and science, community organizations, promising leaders, and the development of innovative technologies. Wendy is president of The Schmidt Family Foundation, which she co-founded with her husband Eric in 2006. They also co-founded Schmidt Ocean Institute and Schmidt Futures.
Editors: The pandemic has altered the course of human history and the nature of our daily lives in equal measure. How has it affected the focus of your philanthropy across your organizations? Have any aspects of the crisis in particular been especially galvanizing as you considered where to concentrate your efforts?
Wendy: The COVID-19 pandemic has made the work of our philanthropy more relevant than ever. If anything, the circumstances of this time have validated the focus we have had for nearly 15 years. We support the need for universal access to clean, renewable energy, healthy food systems, and the dignity of human labor and self-determination in a world of interconnected living systems on land and in the Ocean we are only beginning to understand.
When you consider the disproportionate impact of the COVID-19 virus on people who are poorly paid, poorly housed, with poor nutrition and health care, and exposed to unsafe conditions in the workplace—you see clearly how the systems that have been defining how we live, what we eat, who gets healthcare and what impacts the environment around us—need to change.
"This moment has propelled broad movements toward open publication and open sharing of data and samples—something that has always been a core belief in how we support and advance science."
If the pandemic teaches us anything, we learn what resilience looks like, and the essential role for local small businesses including restaurants, farms and ranches, dairies and fish markets in the long term vitality of communities. There is resonance, local economic benefit, and also accountability in these smaller systems, with shorter supply chains and less vertical integration.
The consolidation of vertically integrated business operations for the sake of global efficiency reveals its essential weakness when supply chains break down and the failure to encourage local economic centers leads to intense systemic disruption and the possibility of collapse.
Editors: For scientists, one significant challenge has been figuring out how to continue research, if at all, during this time of isolation and distancing. Yet, your research vessel Falkor, of the Schmidt Ocean Institute, is still on its expedition exploring the Coral Sea Marine Park in Australia—except now there are no scientists onboard. What was the vessel up to before the pandemic hit? Can you tell us more about how they are continuing to conduct research from afar now and how that's going?
Wendy: We have been extremely fortunate at Schmidt Ocean Institute. When the pandemic hit in March, our research vessel, Falkor, was already months into a year-long program to research unexplored deep sea canyons around Australia and at the Great Barrier Reef. We were at sea, with an Australian science group aboard, carrying on with our mission of exploration, discovery and communication, when we happened upon what we believe to be the world's longest animal—a siphonophore about 150 feet long, spiraling out at a depth of about 2100 feet at the end of a deeper dive in the Ningaloo Canyon off Western Australia. It was the kind of wondrous creature we find so often when we conduct ROV dives in the world's Ocean.
For more than two months this year, Falkor was reportedly the only research vessel in the world carrying on active research at sea. Once we were able to dock and return the science party to shore, we resumed our program at sea offering a scheduled set of now land-based scientists in lockdown in Australia the opportunity to conduct research remotely, taking advantage of the vessel's ship to shore communications, high resolution cameras and live streaming video. It's a whole new world, and quite wonderful in its own way.
Editors: Normally, 10–15 scientists would be aboard such a vessel. Is "remote research" via advanced video technology here to stay? Are there any upsides to this "new normal"?
Wendy: Like all things pandemic, remote research is an adaptation for what would normally occur. Since we are putting safety of the crew and guest scientists at the forefront, we're working to build strong remote connections between our crew, land based scientists and the many robotic tools on board Falkor. There's no substitute for in person work, but what we've developed during the current cruise is a pretty good and productive alternative in a crisis. And what's important is that this critical scientific research into the deep sea is able to continue, despite the pandemic on land.
Editors: Speaking of marine expeditions, you've sponsored two XPRIZE competitions focused on ocean health. Do you think challenge prizes could fill gaps of the global COVID-19 response, for example, to manufacture more testing kits, accelerate the delivery of PPE, or incentivize other areas of need?
Wendy: One challenge we are currently facing is that innovations don't have the funding pathway to scale, so promising ideas by entrepreneurs, researchers, and even major companies are being developed too slowly. Challenge prizes help raise awareness for problems we are trying to solve and attract new people to help solve those problems by giving them a pathway to contribute.
One idea might be for philanthropy to pair prizes and challenges with an "advanced market commitment" where the government commits to a purchase order for the innovation if it meets a certain test. That could be deeply impactful for areas like PPE and the production of testing kits.
Editors: COVID-19 testing, especially, has been sorely needed, here in the U.S. and in developing countries as well as low-income communities. That's why we're so intrigued by your Schmidt Science Fellows grantee Hal Holmes and his work to repurpose a new DNA technology to create a portable, mobile test for COVID-19. Can you tell us about that work and how you are supporting it?
Wendy: Our work with Conservation X Labs began years ago when our foundation was the first to support their efforts to develop a handheld DNA barcode sensor to help detect illegally imported and mislabeled seafood and timber products. The device was developed by Hal Holmes, who became one of our Schmidt Science Fellows and is the technical lead on the project, working closely with Conservation X Labs co-founders Alex Deghan and Paul Bunje. Now, with COVID-19, Hal and team have worked with another Schmidt Science Fellow, Fahim Farzardfard, to repurpose the technology—which requires no continuous power source, special training, or a lab—to serve as a mobile testing device for the virus.
The work is going very well, manufacturing is being organized, and distribution agreements with hospitals and government agencies are underway. You could see this device in use within a few months and have testing results within hours instead of days. It could be especially useful in low-income communities and developing countries where access to testing is challenging.
Editors: How is Schmidt Futures involved in the development of information platforms that will offer productive solutions?
Wendy: In addition to the work I've mentioned, we've also funded the development of tech-enabled tools that can help the medical community be better prepared for the ongoing spike of COVID cases. For example, we funded EdX and Learning Agency to develop an online training to help increase the number of medical professionals who can operate ventilators. The first course is being offered by Harvard University, and so far, over 220,000 medical professionals have enrolled. We have also invested in informational platforms that make it easier to contain the spread of the disease, such as our work with Recidiviz to model the impact of COVID-19 in prisons and outline policy steps states could take to limit the spread.
Information platforms can also play a big part pushing forward scientific research into the virus. For example, we've funded the UC Santa Cruz Virus Browser, which allows researchers to examine each piece of the virus and see the proteins it creates, the interactions in the host cell, and — most importantly — almost everything the recent scientific literature has to say about that stretch of the molecule.
Editors: The scale of research collaboration and the speed of innovation today seem unprecedented. The whole science world has turned its attention to combating the pandemic. What positive big-picture trends do you think or hope will persist once the crisis eventually abates?
Wendy: As in many areas, the COVID crisis has accelerated trends in the scientific world that were already well underway. For instance, this moment has propelled broad movements toward open publication and open sharing of data and samples—something that has always been a core belief in how we support and advance science.
We believe collaboration is an essential ingredient for progress in all areas. Early in this pandemic, Schmidt Futures held a virtual gathering of 160 people across 70 organizations in philanthropy, government, and business interested in accelerating research and response to the virus, and thought at the time, it's pretty amazing this kind of thing doesn't go all the time. We are obviously going to go farther together than on our own...
My husband, Eric, has observed that in the past two months, we've all catapulted 10 years forward in our use of technology, so there are trends already underway that are likely accelerated and will become part of the fabric of the post-COVID world—like working remotely; online learning; increased online shopping, even for groceries; telemedicine; increasing use of AI to create smarter delivery systems for healthcare and many other applications in a world that has grown more virtual overnight.
"Our deepest hope is that out of these alarming and uncertain times will come a renewed appreciation for the tools of science, as they help humans to navigate a world of interconnected living systems, of which viruses are a large part."
We fully expect these trends to continue and expand across the sciences, sped up by the pressures of the health crisis. Schmidt Ocean Institute and Schmidt Futures have been pressing in these directions for years, so we are pleased to see the expansions that should help more scientists work productively, together.
Editors: Trying to find the good amid a horrible crisis, are there any other new horizons in science, philanthropy, and/or your own work that could transform our world for the better that you'd like to share?
Wendy: Our deepest hope is that out of these alarming and uncertain times will come a renewed appreciation for the tools of science, as they help humans to navigate a world of interconnected living systems, of which viruses are a large part. The more we investigate the Ocean, the more we look deeply into what lies in our soils and beneath them, the more we realize we do not know, and moreover, how vulnerable humanity is to the forces of the natural world.
Philanthropy has an important role to play in influencing how people perceive our place in the world and understand the impact of human activity on the rest of the planet. I believe it's philanthropy's role to take risks, to invest early in innovative technologies, to lead where governments and industry aren't ready to go yet. We're fortunate at this time to be able to help those working on tools to better diagnose and treat the virus, and to invest in those working to improve information systems, so citizens and policy makers can make better decisions that can reduce impacts on families and institutions.
From all we know, this isn't likely to be the last pandemic the world will see. It's been said that a crisis comes before change, and we would hope that we can play a role in furthering the work to build systems that are resilient—in information, energy, agriculture and in all the ways we work, recreate, and use the precious resources of our planet.
[This article was originally published on June 8th, 2020 as part of a standalone magazine called GOOD10: The Pandemic Issue. Produced as a partnership among LeapsMag, The Aspen Institute, and GOOD, the magazine is available for free online.]
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.