New Options Are Emerging in the Search for Better Birth Control
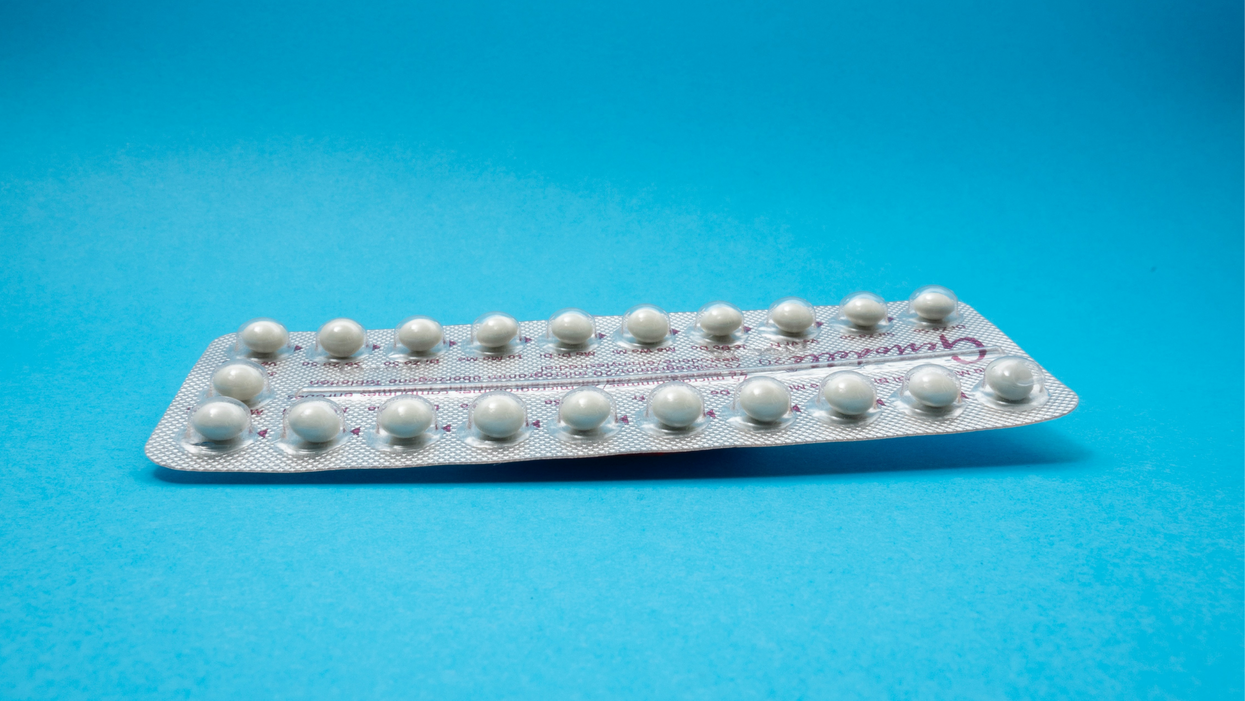
A decade ago, Elizabeth Summers' options for birth control suddenly narrowed. Doctors diagnosed her with Factor V Leiden, a rare genetic disorder, after discovering blood clots in her lungs. The condition increases the risk of clotting, so physicians told Summers to stay away from the pill and other hormone-laden contraceptives. "Modern medicine has generally failed to provide me with an effective and convenient option," she says.
But new birth control options are emerging for women like Summers. These alternatives promise to provide more choices to women who can't ingest hormones or don't want to suffer their unpleasant side effects.
These new products have their own pros and cons. Still, doctors are welcoming new contraceptives following a long drought in innovation. "It's been a long time since we've had something new in the world of contraception," says Heather Irobunda, an obstetrician and gynecologist at NYC Health and Hospitals.
On social media, Irobunda often fields questions about one of these new options, a lubricating gel called Phexxi. San Diego-based Evofem, the company behind Phexxi, has been advertising the product on Hulu and Instagram after the gel was approved by the Food and Drug Administration in May 2020. The company's trendy ads target women who feel like condoms diminish the mood, but who also don't want to mess with an IUD or hormones.
Here's how it works: Phexxi is inserted via a tampon-like device up to an hour before sex. The gel regulates vaginal pH — essentially, the acidity levels — in a range that's inhospitable to sperm. It sounds a lot like spermicide, which is also placed in the vagina prior to sex to prevent pregnancy. But spermicide can damage the vagina's cell walls, which can increase the risk of contracting sexually transmitted diseases.
"Not only is innovation needed, but women want a non-hormonal option."
Phexxi isn't without side effects either. The most common one is vaginal burning, according to a late-stage trial. It's also possible to develop a urinary tract infection while using the product. That same study found that during typical use, Phexxi is about 86 percent effective at preventing pregnancy. The efficacy rate is comparable to condoms but lower than birth control pills (91 percent) and significantly lower than an IUD (99 percent).
Phexxi – which comes in a pack of 12 – represents a tiny but growing part of the birth control market. Pharmacies dispensed more than 14,800 packs from April through June this year, a 65 percent increase over the previous quarter, according to data from Evofem.
"We've been able to demonstrate that not only is innovation needed, but women want a non-hormonal option," says Saundra Pelletier, Evofem's CEO.
Beyond contraception, the company is carrying out late-stage tests to gauge Phexxi's effectiveness at preventing the sexually transmitted infections chlamydia and gonorrhea.

Phexxi is inserted via a tampon-like device up to an hour before sex.
Phexxi
A New Pill
The first birth control pill arrived in 1960, combining the hormones estrogen and progestin to stop sperm from joining with an egg, giving women control over their fertility. Subsequent formulations sought to ease side effects, by way of lower amounts of estrogen. But some women still experience headaches and nausea – or more serious complications like blood clots. On social media, women recently noted that birth control pills are much more likely to cause blood clots than Johnson & Johnson's COVID-19 vaccine that was briefly paused to evaluate the risk of clots in women under age 50. What will it take, they wondered, for safer birth control?
Mithra Pharmaceuticals of Belgium sought to create a gentler pill. In April, the FDA approved Mithra's Nextstellis, which includes a naturally occurring estrogen, the first new estrogen in the U.S. in 50 years. Nextstellis selectively acts on tissues lining the uterus, while other birth control pills have a broader target.
A Phase 3 trial showed a 98 percent efficacy rate. Andrew London, an obstetrician and gynecologist, who practices at several Maryland hospitals, says the results are in line with some other birth control pills. But, he added, early studies indicate that Nextstellis has a lower risk of blood clotting, along with other potential benefits, which additional clinical testing must confirm.
"It's not going to be worse than any other pill. We're hoping it's going to be significantly better," says London.
The estrogen in Nexstellis, called estetrol, was skipped over by the pharmaceutical industry after its discovery in the 1960s. Estetrol circulates between the mother and fetus during pregnancy. Decades later, researchers took a new look, after figuring out how to synthesize estetrol in a lab, as well as produce estetrol from plants.
"That allowed us to really start to investigate the properties and do all this stuff you have to do for any new drug," says Michele Gordon, vice president of marketing in women's health at Mayne Pharma, which licensed Nextstellis.
Bonnie Douglas, who followed the development of Nextstellis as part of a search for better birth control, recently switched to the product. "So far, it's much more tolerable," says Douglas. Previously, the Midwesterner was so desperate to find a contraceptive with fewer side effects that she turned to an online pharmacy to obtain a different birth control pill that had been approved in Canada but not in the U.S.
Contraceptive Access
Even if a contraceptive lands FDA approval, access poses a barrier. Getting insurers to cover new contraceptives can be difficult. For the uninsured, state and federal programs can help, and companies should keep prices in a reasonable range, while offering assistance programs. So says Kelly Blanchard, president of the nonprofit Ibis Reproductive Health. "For innovation to have impact, you want to reach as many folks as possible," she says.
In addition, companies developing new contraceptives have struggled to attract venture capital. That's changing, though.
In 2015, Sabrina Johnson founded DARÉ Bioscience around the idea of women's health. She estimated the company would be fully funded in six months, based on her track record in biotech and the demand for novel products.
But it's been difficult to get male investors interested in backing new contraceptives. It took Johnson two and a half years to raise the needed funds, via a reverse merger that took the company public. "There was so much education that was necessary," Johnson says, adding: "The landscape has changed considerably."
Johnson says she would like to think DARÉ had something to do with the shift, along with companies like Organon, a spinout of pharma company Merck that's focused on reproductive health. In surveying the fertility landscape, DARÉ saw limited non-hormonal options. On-demand options – like condoms – can detract from the moment. Copper IUDs must be inserted by a doctor and removed if a woman wants to return to fertility, and this method can have onerous side effects.
So, DARÉ created Ovaprene, a hormone-free device that's designed to be inserted into the vagina monthly by the user. The mesh product acts as a barrier, while releasing a chemical that immobilizes sperm. In an early study, the company reported that Ovaprene prevented almost all sperm from entering the cervical canal. The results, DARÉ believes, indicate high efficacy.
A late-stage study, slated to kick off next year, will be the true judge. Should Ovaprene eventually win regulatory approval, drug giant Bayer will handle commercializing the device.
Other new forms of birth control in development are further out, and that's assuming they perform well in clinical trials. Among them: a once-a-month birth control pill, along with a male version of the birth control pill. The latter is often brought up among women who say it's high time that men take a more proactive role in birth control.
For Summers, her search for a safe and convenient birth control continues. She tried Phexxi, which caused irritation. Still, she's excited that a non-hormonal option now exists. "I'm sure it will work for others," she says.
How Excessive Regulation Helped Ignite COVID-19's Rampant Spread
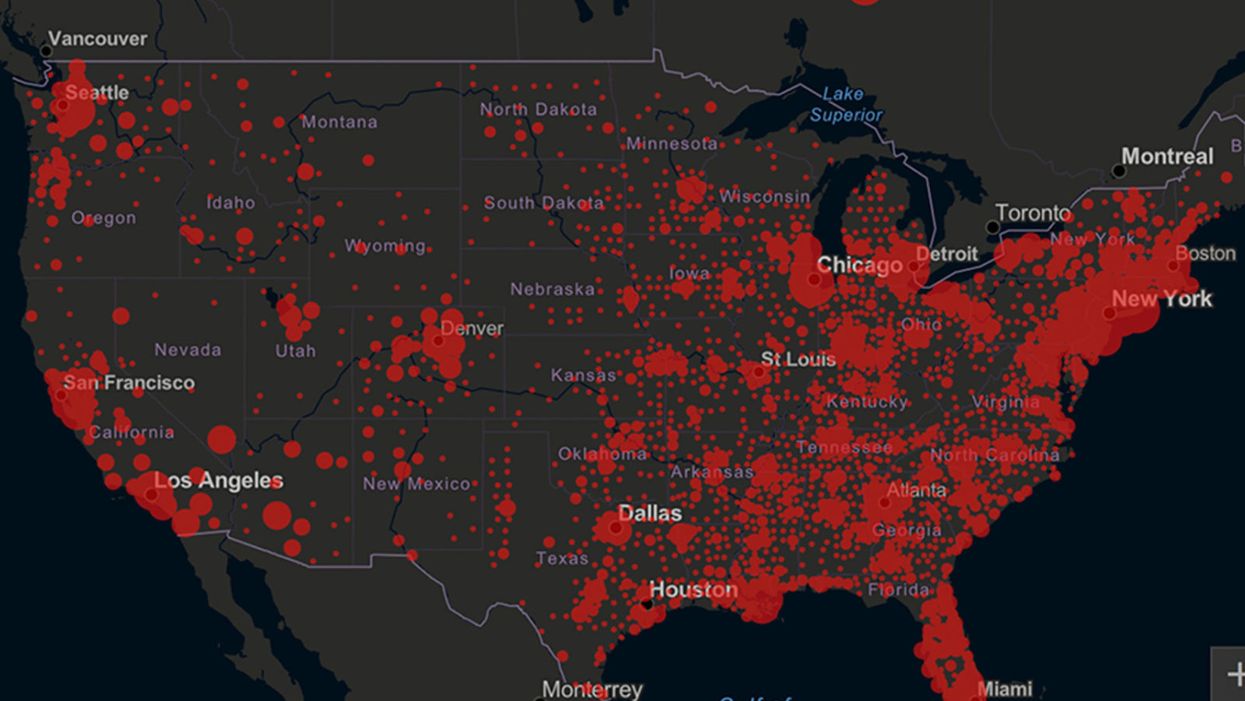
Screenshot of an interactive map of coronavirus cases across the United States, current as of 1:45 p.m. Pacific time on Tuesday, March 31st. Full map accessible at https://coronavirus.jhu.edu/map.html
When historians of the future look back at the 2020 pandemic, the heroic work of Helen Y. Chu, a flu researcher at the University of Washington, will be worthy of recognition.
Chu's team bravely defied the order and conducted the testing anyway.
In late January, Chu was testing nasal swabs for the Seattle Flu Study to monitor influenza spread when she learned of the first case of COVID-19 in Washington state. She deemed it a pressing public health matter to document if and how the illness was spreading locally, so that early containment efforts could succeed. So she sought regulatory approval to adapt the Flu Study to test for the coronavirus, but the federal government denied the request because the original project was funded to study only influenza.
Aware of the urgency, Chu's team bravely defied the order and conducted the testing anyway. Soon they identified a local case in a teenager without any travel history, followed by others. Still, the government tried to shutter their efforts until the outbreak grew dangerous enough to command attention.
Needless testing delays, prompted by excessive regulatory interference, eliminated any chances of curbing the pandemic at its initial stages. Even after Chu went out on a limb to sound alarms, a heavy-handed bureaucracy crushed the nation's ability to roll out early and widespread testing across the country. The Centers for Disease Control and Prevention infamously blundered its own test, while also impeding state and private labs from coming on board, fueling a massive shortage.
The long holdup created "a backlog of testing that needed to be done," says Amesh Adalja, an infectious disease specialist who is a senior scholar at the Johns Hopkins University Center for Health Security.
In a public health crisis, "the ideal situation" would allow the government's test to be "supplanted by private laboratories" without such "a lag in that transition," Adalja says. Only after the eventual release of CDC's test could private industry "begin in earnest" to develop its own versions under the Food and Drug Administration's emergency use authorization.
In a statement, CDC acknowledged that "this process has not gone as smoothly as we would have liked, but there is currently no backlog for testing at CDC."
Now, universities and corporations are in a race against time, playing catch up as the virus continues its relentless spread, also afflicting many health care workers on the front lines.
"Home-testing accessibility is key to preventing further spread of the COVID-19 pandemic."
Hospitals are attempting to add the novel coronavirus to the testing panel of their existent diagnostic machines, which would reduce the results processing time from 48 hours to as little as four hours. Meanwhile, at least four companies announced plans to deliver at-home collection tests to help meet the demand – before a startling injunction by the FDA halted their plans.
Everlywell, an Austin, Texas-based digital health company, had been set to launch online sales of at-home collection kits directly to consumers last week. Scaling up in a matter of days to an initial supply of 30,000 tests, Everlywell collaborated with multiple laboratories where consumers could ship their nasal swab samples overnight, projecting capacity to screen a quarter-million individuals on a weekly basis, says Frank Ong, chief medical and scientific officer.
Secure digital results would have been available online within 48 hours of a sample's arrival at the lab, as well as a telehealth consultation with an independent, board-certified doctor if someone tested positive, for an inclusive $135 cost. The test has a less than 3 percent false-negative rate, Ong says, and in the event of an inadequate self-swab, the lab would not report a conclusive finding. "Home-testing accessibility," he says, "is key to preventing further spread of the COVID-19 pandemic."
But on March 20, the FDA announced restrictions on home collection tests due to concerns about accuracy. The agency did note "the public health value in expanding the availability of COVID-19 testing through safe and accurate tests that may include home collection," while adding that "we are actively working with test developers in this space."
After the restrictions were announced, Everlywell decided to allocate its initial supply of COVID-19 collection kits to hospitals, clinics, nursing homes, and other qualifying health care companies that can commit to no-cost screening of frontline workers and high-risk symptomatic patients. For now, no consumers can order a home-collection test.
"Losing two months is close to disastrous, and that's what we did."
Currently, the U.S. has ramped up to testing an estimated 100,000 people a day, according to Stat News. But 150,000 or more Americans should be tested every day, says Ashish Jha, professor and director of the Harvard Global Health Institute. Due to the dearth of tests, many sick people who suspect they are infected still cannot get confirmation unless they need to be hospitalized.
To give a concrete sense of how far behind we are in testing, consider Palm Beach County, Fla. The state's only drive-thru test center just opened there, requiring an appointment. The center aims to test 750 people per day, but more than 330,000 people have already called to try to book a slot.
"This is such a rapidly moving infection that losing a few days is bad, and losing a couple of weeks is terrible," says Jha, a practicing general internist. "Losing two months is close to disastrous, and that's what we did."
At this point, it will take a long time to fully ramp up. "We are blindfolded," he adds, "and I'd like to take the blindfolds off so we can fight this battle with our eyes wide open."
Better late than never: Yesterday, FDA Commissioner Stephen Hahn said in a statement that the agency has worked with more than 230 test developers and has approved 20 tests since January. An especially notable one was authorized last Friday – 67 days since the country's first known case in Washington state. It's a rapid point-of-care test from medical-device firm Abbott that provides positive results in five minutes and negative results in 13 minutes. Abbott will send 50,000 tests a day to urgent care settings. The first tests are expected to ship tomorrow.
Your Privacy vs. the Public's Health: High-Tech Tracking to Fight COVID-19 Evokes Orwell
Governments around the world are using technology to track their citizens to contain COVID-19.
The COVID-19 pandemic has placed public health and personal privacy on a collision course, as smartphone technology has completely rewritten the book on contact tracing.
It's not surprising that an autocratic regime like China would adopt such measures, but democracies such as Israel have taken a similar path.
The gold standard – patient interviews and detective work – had been in place for more than a century. It's been all but replaced by GPS data in smartphones, which allows contact tracing to occur not only virtually in real time, but with vastly more precision.
China has gone the furthest in using such tech to monitor and prevent the spread of the coronavirus. It developed an app called Health Code to determine which of its citizens are infected or at risk of becoming infected. It has assigned each individual a color code – red, yellow or green – and restricts their movement depending on their assignment. It has also leveraged its millions of public video cameras in conjunction with facial recognition tech to identify people in public who are not wearing masks.
It's not surprising that an autocratic regime like China would adopt such measures, but democracies such as Israel have taken a similar path. The national security agency Shin Bet this week began analyzing all personal cellphone data under emergency measures approved by the government. It texts individuals when it's determined they had been in contact with someone who had the coronavirus. In Spain and China, police have sent drones aloft searching for people violating stay-at-home orders. Commands to disperse can be issued through audio systems built into the aircraft. In the U.S., efforts are underway to lift federal restrictions on drones so that police can use them to prevent people from gathering.
The chief executive of a drone manufacturer in the U.S. aptly summed up the situation in an interview with the Financial Times: "It seems a little Orwellian, but this could save lives."
Epidemics and how they're surveilled often pose thorny dilemmas, according to Craig Klugman, a bioethicist and professor of health sciences at DePaul University in Chicago. "There's always a moral issue to contact tracing," he said, adding that the issue doesn't change by nation, only in the way it's resolved.
"Once certain privacy barriers have been breached, it can be difficult to roll them back again."
In China, there's little to no expectation for privacy, so their decision to take the most extreme measures makes sense to Klugman. "In China, the community comes first. In the U.S., individual rights come first," he said.
As the U.S. has scrambled to develop testing kits and manufacture ventilators to identify potential patients and treat them, individual rights have mostly not received any scrutiny. However, that could change in the coming weeks.
The American approach is also leaning toward using smartphone apps, but in a way that may preserve the privacy of users. Researchers at MIT have released a prototype known as Private Kit: Safe Paths. Patients diagnosed with the coronavirus can use the app to disclose their location trail for the prior 28 days to other users without releasing their specific identity. They also have the option of sharing the data with public health officials. But such an app would only be effective if there is a significant number of users.
Singapore is offering a similar app to its citizens known as TraceTogether, which uses both GPS and Bluetooth pings among users to trace potential encounters. It's being offered on a voluntary basis.
The Electronic Frontier Foundation, the leading nonprofit organization defending civil liberties in the digital world, said it is monitoring how these apps are developed and deployed. "Governments around the world are demanding new dragnet location surveillance powers to contain the COVID-19 outbreak," it said in a statement. "But before the public allows their governments to implement such systems, governments must explain to the public how these systems would be effective in stopping the spread of COVID-19. There's no questioning the need for far-reaching public health measures to meet this urgent challenge, but those measures must be scientifically rigorous, and based on the expertise of public health professionals."
Andrew Geronimo, director of the intellectual property venture clinic at the Case Western University School of Law, said that the U.S. government is currently in talks with Facebook, Google and other tech companies about using deidentified location data from smartphones to better monitor the progress of the outbreak. He was hesitant to endorse such a step.
"These companies may say that all of this data is anonymized," he said, "but studies have shown that it is difficult to fully anonymize data sets that contain so much information about us."
Beyond the technical issues, social attitudes may mount another challenge. Epic events such as 9/11 tend to loosen vigilance toward protecting privacy, according to Klugman and Geronimo. And as more people are sickened and hospitalized in the U.S. with COVID-19, Klugman believes more Americans will be willing to allow themselves to be tracked. "If that happens, there needs to be a time limitation," he said.
However, even if time limits are put in place, Geronimo believes it would lead to an even greater rollback of privacy during the next crisis.
"Once certain privacy barriers have been breached, it can be difficult to roll them back again," he warned. "And the prior incidents could always be used as a precedent – or as proof of concept."