New Options Are Emerging in the Search for Better Birth Control

A decade ago, Elizabeth Summers' options for birth control suddenly narrowed. Doctors diagnosed her with Factor V Leiden, a rare genetic disorder, after discovering blood clots in her lungs. The condition increases the risk of clotting, so physicians told Summers to stay away from the pill and other hormone-laden contraceptives. "Modern medicine has generally failed to provide me with an effective and convenient option," she says.
But new birth control options are emerging for women like Summers. These alternatives promise to provide more choices to women who can't ingest hormones or don't want to suffer their unpleasant side effects.
These new products have their own pros and cons. Still, doctors are welcoming new contraceptives following a long drought in innovation. "It's been a long time since we've had something new in the world of contraception," says Heather Irobunda, an obstetrician and gynecologist at NYC Health and Hospitals.
On social media, Irobunda often fields questions about one of these new options, a lubricating gel called Phexxi. San Diego-based Evofem, the company behind Phexxi, has been advertising the product on Hulu and Instagram after the gel was approved by the Food and Drug Administration in May 2020. The company's trendy ads target women who feel like condoms diminish the mood, but who also don't want to mess with an IUD or hormones.
Here's how it works: Phexxi is inserted via a tampon-like device up to an hour before sex. The gel regulates vaginal pH — essentially, the acidity levels — in a range that's inhospitable to sperm. It sounds a lot like spermicide, which is also placed in the vagina prior to sex to prevent pregnancy. But spermicide can damage the vagina's cell walls, which can increase the risk of contracting sexually transmitted diseases.
"Not only is innovation needed, but women want a non-hormonal option."
Phexxi isn't without side effects either. The most common one is vaginal burning, according to a late-stage trial. It's also possible to develop a urinary tract infection while using the product. That same study found that during typical use, Phexxi is about 86 percent effective at preventing pregnancy. The efficacy rate is comparable to condoms but lower than birth control pills (91 percent) and significantly lower than an IUD (99 percent).
Phexxi – which comes in a pack of 12 – represents a tiny but growing part of the birth control market. Pharmacies dispensed more than 14,800 packs from April through June this year, a 65 percent increase over the previous quarter, according to data from Evofem.
"We've been able to demonstrate that not only is innovation needed, but women want a non-hormonal option," says Saundra Pelletier, Evofem's CEO.
Beyond contraception, the company is carrying out late-stage tests to gauge Phexxi's effectiveness at preventing the sexually transmitted infections chlamydia and gonorrhea.

Phexxi is inserted via a tampon-like device up to an hour before sex.
Phexxi
A New Pill
The first birth control pill arrived in 1960, combining the hormones estrogen and progestin to stop sperm from joining with an egg, giving women control over their fertility. Subsequent formulations sought to ease side effects, by way of lower amounts of estrogen. But some women still experience headaches and nausea – or more serious complications like blood clots. On social media, women recently noted that birth control pills are much more likely to cause blood clots than Johnson & Johnson's COVID-19 vaccine that was briefly paused to evaluate the risk of clots in women under age 50. What will it take, they wondered, for safer birth control?
Mithra Pharmaceuticals of Belgium sought to create a gentler pill. In April, the FDA approved Mithra's Nextstellis, which includes a naturally occurring estrogen, the first new estrogen in the U.S. in 50 years. Nextstellis selectively acts on tissues lining the uterus, while other birth control pills have a broader target.
A Phase 3 trial showed a 98 percent efficacy rate. Andrew London, an obstetrician and gynecologist, who practices at several Maryland hospitals, says the results are in line with some other birth control pills. But, he added, early studies indicate that Nextstellis has a lower risk of blood clotting, along with other potential benefits, which additional clinical testing must confirm.
"It's not going to be worse than any other pill. We're hoping it's going to be significantly better," says London.
The estrogen in Nexstellis, called estetrol, was skipped over by the pharmaceutical industry after its discovery in the 1960s. Estetrol circulates between the mother and fetus during pregnancy. Decades later, researchers took a new look, after figuring out how to synthesize estetrol in a lab, as well as produce estetrol from plants.
"That allowed us to really start to investigate the properties and do all this stuff you have to do for any new drug," says Michele Gordon, vice president of marketing in women's health at Mayne Pharma, which licensed Nextstellis.
Bonnie Douglas, who followed the development of Nextstellis as part of a search for better birth control, recently switched to the product. "So far, it's much more tolerable," says Douglas. Previously, the Midwesterner was so desperate to find a contraceptive with fewer side effects that she turned to an online pharmacy to obtain a different birth control pill that had been approved in Canada but not in the U.S.
Contraceptive Access
Even if a contraceptive lands FDA approval, access poses a barrier. Getting insurers to cover new contraceptives can be difficult. For the uninsured, state and federal programs can help, and companies should keep prices in a reasonable range, while offering assistance programs. So says Kelly Blanchard, president of the nonprofit Ibis Reproductive Health. "For innovation to have impact, you want to reach as many folks as possible," she says.
In addition, companies developing new contraceptives have struggled to attract venture capital. That's changing, though.
In 2015, Sabrina Johnson founded DARÉ Bioscience around the idea of women's health. She estimated the company would be fully funded in six months, based on her track record in biotech and the demand for novel products.
But it's been difficult to get male investors interested in backing new contraceptives. It took Johnson two and a half years to raise the needed funds, via a reverse merger that took the company public. "There was so much education that was necessary," Johnson says, adding: "The landscape has changed considerably."
Johnson says she would like to think DARÉ had something to do with the shift, along with companies like Organon, a spinout of pharma company Merck that's focused on reproductive health. In surveying the fertility landscape, DARÉ saw limited non-hormonal options. On-demand options – like condoms – can detract from the moment. Copper IUDs must be inserted by a doctor and removed if a woman wants to return to fertility, and this method can have onerous side effects.
So, DARÉ created Ovaprene, a hormone-free device that's designed to be inserted into the vagina monthly by the user. The mesh product acts as a barrier, while releasing a chemical that immobilizes sperm. In an early study, the company reported that Ovaprene prevented almost all sperm from entering the cervical canal. The results, DARÉ believes, indicate high efficacy.
A late-stage study, slated to kick off next year, will be the true judge. Should Ovaprene eventually win regulatory approval, drug giant Bayer will handle commercializing the device.
Other new forms of birth control in development are further out, and that's assuming they perform well in clinical trials. Among them: a once-a-month birth control pill, along with a male version of the birth control pill. The latter is often brought up among women who say it's high time that men take a more proactive role in birth control.
For Summers, her search for a safe and convenient birth control continues. She tried Phexxi, which caused irritation. Still, she's excited that a non-hormonal option now exists. "I'm sure it will work for others," she says.
A simple ten-minute universal cancer test that can be detected by the human eye or an electronic device - published in Nature Communications (Dec 2018) by the Trau lab at the University of Queensland. Red indicates the presence of cancerous cells and blue doesn't.
Matt Trau, a professor of chemistry at the University of Queensland, stunned the science world back in December when the prestigious journal Nature Communications published his lab's discovery about a unique property of cancer DNA that could lead to a simple, cheap, and accurate test to detect any type of cancer in under 10 minutes.
No one believed it. I didn't believe it. I thought, "Gosh, okay, maybe it's a fluke."
Trau granted very few interviews in the wake of the news, but he recently opened up to leapsmag about the significance of this promising early research. Here is his story in his own words, as told to Editor-in-Chief Kira Peikoff.
There's been an incredible explosion of knowledge over the past 20 years, particularly since the genome was sequenced. The area of diagnostics has a tremendous amount of promise and has caught our lab's interest. If you catch cancer early, you can improve survival rates to as high as 98 percent, sometimes even now surpassing that.
My lab is interested in devices to improve the trajectory of cancer patients. So, once people get diagnosed, can we get really sophisticated information about the molecular origins of the disease, and can we measure it in real time? And then can we match that with the best treatment and monitor it in real time, too?
I think those approaches, also coupled with immunotherapy, where one dreams of monitoring the immune system simultaneously with the disease progress, will be the future.
But currently, the methodologies for cancer are still pretty old. So, for example, let's talk about biopsies in general. Liquid biopsy just means using a blood test or a urine test, rather than extracting out a piece of solid tissue. Now consider breast cancer. Still, the cutting-edge screening method is mammography or the physical interrogation for lumps. This has had a big impact in terms of early detection and awareness, but it's still primitive compared to interrogating, forensically, blood samples to look at traces of DNA.
Large machines like CAT scans, PET scans, MRIs, are very expensive and very subjective in terms of the operator. They don't look at the root causes of the cancer. Cancer is caused by changes in DNA. These can be changes in the hard drive of the DNA (the genomic changes) or changes in the apps that the DNA are running (the epigenetics and the transcriptomics).
We don't look at that now, even though we have, emerging, all of these technologies to do it, and those technologies are getting so much cheaper. I saw some statistics at a conference just a few months ago that, in the United States, less than 1 percent of cancer patients have their DNA interrogated. That's the current state-of-the-art in the modern medical system.

Professor Matt Trau, a cancer researcher at the University of Queensland in Australia.
(Courtesy)
Blood, as the highway of the body, is carrying all of this information. Cancer cells, if they are present in the body, are constantly getting turned over. When they die, they release their contents into the blood. Many of these cells end up in the urine and saliva. Having technologies that can forensically scan the highways looking for evidence of cancer is little bit like looking for explosives at the airport. That's very valuable as a security tool.
The trouble is that there are thousands of different types of cancer. Going back to breast cancer, there's at least a dozen different types, probably more, and each of them change the DNA (the hard drive of the disease) and the epigenetics (or the RAM memory). So one of the problems for diagnostics in cancer is to find something that is a signature of all cancers. That's been a really, really, really difficult problem.
Ours was a completely serendipitous discovery. What we found in the lab was this one marker that just kept coming up in all of the types of breast cancers we were studying.
No one believed it. I didn't believe it. I thought, "Gosh, okay, maybe it's a fluke, maybe it works just for breast cancer." So we went on to test it in prostate cancer, which is also many different types of diseases, and it seemed to be working in all of those. We then tested it further in lymphoma. Again, many different types of lymphoma. It worked across all of those. We tested it in gastrointestinal cancer. Again, many different types, and still, it worked, but we were skeptical.
Then we looked at cell lines, which are cells that have come from previous cancer patients, that we grow in the lab, but are used as model experimental systems. We have many of those cell lines, both ones that are cancerous, and ones that are healthy. It was quite remarkable that the marker worked in all of the cancer cell lines and didn't work in the healthy cell lines.
What could possibly be going on?
Well, imagine DNA as a piece of string, that's your hard drive. Epigenetics is like the beads that you put on that string. Those beads you can take on and off as you wish and they control which apps are run, meaning which genetic programs the cell runs. We hypothesized that for cancer, those beads cluster together, rather than being randomly distributed across the string.
Ultimately, I see this as something that would be like a pregnancy test you could take at your doctor's office.
The implications of this are profound. It means that DNA from cancer folds in water into three-dimensional structures that are very different from healthy cells' DNA. It's quite literally the needle in a haystack. Because when you do a liquid biopsy for early detection of cancer, most of the DNA from blood contains a vast abundance of healthy DNA. And that's not of interest. What's of interest is to find the cancerous DNA. That's there only in trace.
Once we figured out what was going on, we could easily set up a system to detect the trace cancerous DNA. It binds to gold nanoparticles in water and changes color. The test takes 10 minutes, and you can detect it by eye. Red indicates cancer and blue doesn't.
We're very, very excited about where we go from here. We're starting to test the test on a greater number of cancers, in thousands of patient samples. We're looking to the scientific community to engage with us, and we're getting a really good response from groups around the world who are supplying more samples to us so we can test this more broadly.
We also are very interested in testing how early can we go with this test. Can we detect cancer through a simple blood test even before there are any symptoms whatsoever? If so, we might be able to convert a cancer diagnosis to something almost as good as a vaccine.
Of course, we have to watch what are called false positives. We don't want to be detecting people as positives when they don't have cancer, and so the technology needs to improve there. We see this version as the iPhone 1. We're interested in the iPhone 2, 3, 4, getting better and better.
Ultimately, I see this as something that would be like a pregnancy test you could take at your doctor's office. If it came back positive, your doctor could say, "Look, there's some news here, but actually, it's not bad news, it's good news. We've caught this so early that we will be able to manage this, and this won't be a problem for you."
If this were to be in routine use in the medical system, countless lives could be saved. Cancer is now becoming one of the biggest killers in the world. We're talking millions upon millions upon millions of people who are affected. This really motivates our work. We might make a difference there.
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
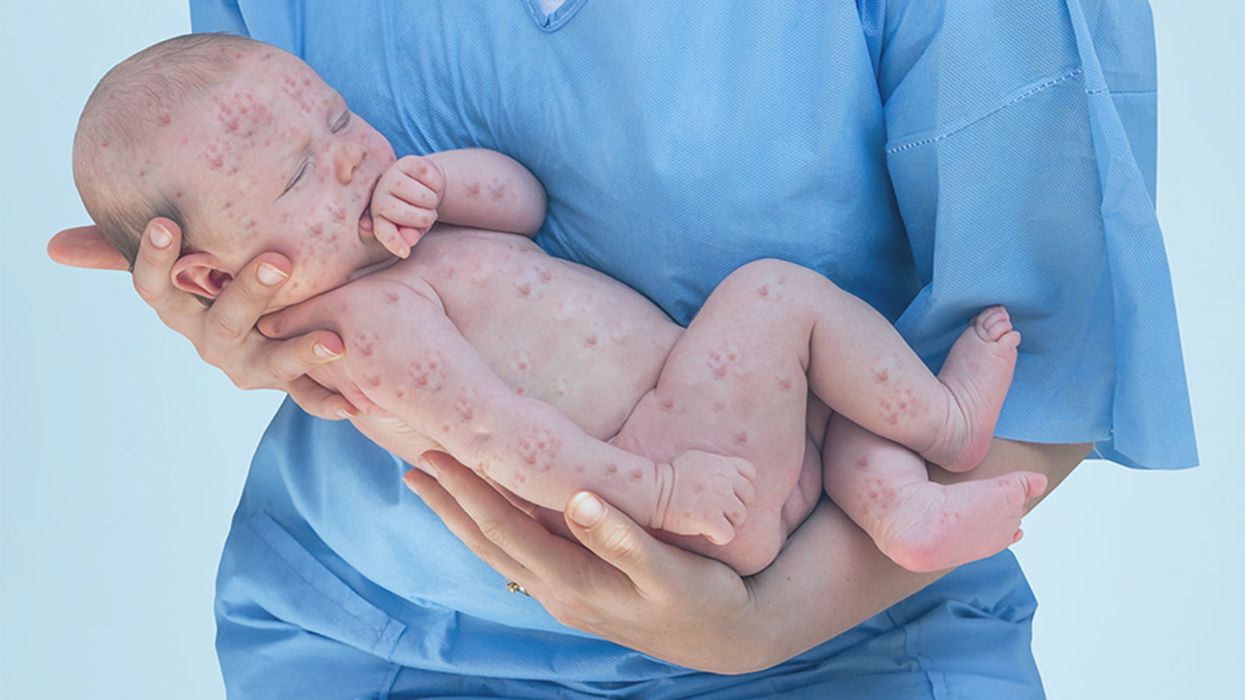
A doctor cradles a newborn who is sick with measles.
Ethan Lindenberger, the Ohio teenager who sought out vaccinations after he was denied them as a child, recently testified before Congress about why his parents became anti-vaxxers. The trouble, he believes, stems from the pervasiveness of misinformation online.
There is evidence that 'educating' people with facts about the benefits of vaccination may not be effective.
"For my mother, her love and affection and care as a parent was used to push an agenda to create a false distress," he told the Senate Committee. His mother read posts on social media saying vaccines are dangerous, and that was enough to persuade her against them.
His story is an example of how widespread and harmful the current discourse on vaccinations is—and more importantly—how traditional strategies to convince people about the merits of vaccination have largely failed.
As responsible members of society, all of us have implicitly signed on to what ethicists call the "Social Contract" -- we agree to abide by certain moral and political rules of behavior. This is what our societal values, norms, and often governments are based upon. However, with the unprecedented rise of social media, alternative facts, and fake news, it is evident that our understanding—and application—of the social contract must also evolve.
Nowhere is this breakdown of societal norms more visible than in the failure to contain the spread of vaccine-preventable diseases like measles. What started off as unexplained episodes in New York City last October, mostly in communities that are under-vaccinated, has exploded into a national epidemic: 880 cases of measles across 24 states in 2019, according to the CDC (as of May 17, 2019). In fact, the Unites States is only eight months away from losing its "measles free" status, joining Venezuela as the second country out of North and South America with that status.
The U.S. is not the only country facing this growing problem. Such constant and perilous reemergence of measles and other vaccine-preventable diseases in various parts of the world raises doubts about the efficacy of current vaccination policies. In addition to the loss of valuable life, these outbreaks lead to loss of millions of dollars in unnecessary expenditure of scarce healthcare resources. While we may be living through an age of information, we are also navigating an era whose hallmark is a massive onslaught on truth.
There is ample evidence on how these outbreaks start: low-vaccination rates. At the same time, there is evidence that 'educating' people with facts about the benefits of vaccination may not be effective. Indeed, human reasoning has a limit, and facts alone rarely change a person's opinion. In a fascinating report by researchers from the University of Pennsylvania, a small experiment revealed how "behavioral nudges" could inform policy decisions around vaccination.
In the reported experiment, the vaccination rate for employees of a company increased by 1.5 percent when they were prompted to name the date when they planned to get their flu shot. In the same experiment, when employees were prompted to name both a date and a time for their planned flu shot, vaccination rate increased by 4 percent.
A randomized trial revealed the subtle power of "announcements" – direct, brief, assertive statements by physicians that assumed parents were ready to vaccinate their children.
This experiment is a part of an emerging field of behavioral economics—a scientific undertaking that uses insights from psychology to understand human decision-making. The field was born from a humbling realization that humans probably do not possess an unlimited capacity for processing information. Work in this field could inform how we can formulate vaccination policy that is effective, conserves healthcare resources, and is applicable to current societal norms.
Take, for instance, the case of Human Papilloma Virus (HPV) that can cause several types of cancers in both men and women. Research into the quality of physician communication has repeatedly revealed how lukewarm recommendations for HPV vaccination by primary care physicians likely contributes to under-immunization of eligible adolescents and can cause confusion for parents.
A randomized trial revealed the subtle power of "announcements" – direct, brief, assertive statements by physicians that assumed parents were ready to vaccinate their children. These announcements increased vaccination rates by 5.4 percent. Lengthy, open-ended dialogues demonstrated no benefit in vaccination rates. It seems that uncertainty from the physician translates to unwillingness from a parent.
Choice architecture is another compelling concept. The premise is simple: We hardly make any of our decisions in vacuum; the environment in which these decisions are made has an influence. If health systems were designed with these insights in mind, people would be more likely to make better choices—without being forced.
This theory, proposed by Richard Thaler, who won the 2017 Nobel Prize in Economics, was put to the test by physicians at the University of Pennsylvania. In their study, flu vaccination rates at primary care practices increased by 9.5 percent all because the staff implemented "active choice intervention" in their electronic health records—a prompt that nudged doctors and nurses to ask patients if they'd gotten the vaccine yet. This study illustrated how an intervention as simple as a reminder can save lives.
To be sure, some bioethicists do worry about implementing these policies. Are behavioral nudges akin to increased scrutiny or a burden for the disadvantaged? For example, would incentives to quit smoking unfairly target the poor, who are more likely to receive criticism for bad choices?
The measles outbreak is a sober reminder of how devastating it can be when the social contract breaks down.
While this is a valid concern, behavioral economics offers one of the only ethical solutions to increasing vaccination rates by addressing the most critical—and often legal—challenge to universal vaccinations: mandates. Choice architecture and other interventions encourage and inform a choice, allowing an individual to retain his or her right to refuse unwanted treatment. This distinction is especially important, as evidence suggests that people who refuse vaccinations often do so as a result of cognitive biases – systematic errors in thinking resulting from emotional attachment or a lack of information.
For instance, people are prone to "confirmation bias," or a tendency to selectively believe in information that confirms their preexisting theories, rather than the available evidence. At the same time, people do not like mandates. In such situations, choice architecture provides a useful option: people are nudged to make the right choice via the design of health delivery systems, without needing policies that rely on force.
The measles outbreak is a sober reminder of how devastating it can be when the social contract breaks down and people fall prey to misinformation. But all is not lost. As we fight a larger societal battle against alternative facts, we now have another option in the trenches to subtly encourage people to make better choices.
Using insights from research in decision-making, we can all contribute meaningfully in controversial conversations with family, friends, neighbors, colleagues, and our representatives — and push for policies that protect those we care about. A little more than a hundred years ago, thousands of lives were routinely lost to preventive illnesses. We've come too far to let ignorance destroy us now.