New Hope for Organ Transplantation: Life Without Anti-Rejection Drugs

Kidney transplant patient Robert Waddell, center, with his wife and children after being off immunosuppresants; photo aken last summer in Perdido Key, FL. Left to right: Christian, Bailey, Rob, Karen (wife), Robby and Casey.
Rob Waddell dreaded getting a kidney transplant. He suffers from a genetic condition called polycystic kidney disease that causes the uncontrolled growth of cysts that gradually choke off kidney function. The inherited defect has haunted his family for generations, killing his great grandmother, grandmother, and numerous cousins, aunts and uncles.
But he saw how difficult it was for his mother and sister, who also suffer from this condition, to live with the side effects of the drugs they needed to take to prevent organ rejection, which can cause diabetes, high blood pressure and cancer, and even kidney failure because of their toxicity. Many of his relatives followed the same course, says Waddell: "They were all on dialysis, then a transplant and ended up usually dying from cancers caused by the medications."
When the Louisville native and father of four hit 40, his kidneys barely functioned and the only alternative was either a transplant or the slow death of dialysis. But in 2009, when Waddell heard about an experimental procedure that could eliminate the need for taking antirejection drugs, he jumped at the chance to be their first patient. Devised by scientists at the University of Louisville and Northwestern University, the innovative approach entails mixing stem cells from the live kidney donor with that of the recipient to create a hybrid immune system, known as a chimera, that would trick the immune system and prevent it from attacking the implanted kidney.
The procedure itself was done at Northwestern Memorial Hospital in Chicago, using a live kidney donated by a neighbor of Waddell's, who camped out in Chicago during his recovery. Prior to surgery, Waddell underwent a conditioning treatment that consisted of low dose radiation and chemotherapy to weaken his own immune system and make room for the infusion of stem cells.
"The low intensity chemo and radiation conditioning regimen create just enough space for the donor stem cells to gain a foothold in the bone marrow and the donor's immune system takes over," says Dr. Joseph Levanthal, the transplant surgeon who performed the operation and director of kidney and pancreas transplantation at Northwestern University Feinberg School of Medicine. "That way the recipient develops an immune system that doesn't see the donor organ as foreign."
"As a surgeon, I saw what my patients had to go through—taking 25 pills a day, dying at an early age from heart disease, or having a 35% chance of dying every year on dialysis."
A week later, Waddell had the kidney transplant. The following day, he was infused with a complex cellular cocktail that included blood-forming stem cells derived from his donor's bone marrow mixed what are called tolerance inducing facilitator cells (FCs); these cells help the foreign stem cells get established in the recipient's bone marrow.
Over the course of the following year, he was slowly weaned off of antirejection medications—a precaution in case the procedure didn't work—and remarkably, hasn't needed them since. "I felt better than I had in decades because my kidneys [had been] degrading," recalls Waddell, now 54 and a CPA for a global beverage company. And what's even better is that this new approach offers hope for one of his sons who has also inherited the disorder.
Kidney transplants are the most frequent organ transplants in the world and more than 23,000 of these procedures were done in the United States in 2019, according to the United Network for Organ Sharing. Of this, about 7,000 operations are done annually using live organ donors; the remainder use organs from people who are deceased. Right now, this revolutionary new approach—as well as a similar strategy formulated by Stanford University scientists--is in the final phase of clinical trials. Ultimately, this research may pave the way towards realizing the holy grail of organ transplantation: preventing organ rejection by creating a tolerant state in which the recipient's immune system is compatible with the donor, which would eliminate the need for a lifetime of medications.
"As a surgeon, I saw what my patients had to go through—taking 25 pills a day, dying at an early age from heart disease, or having a 35% chance of dying every year on dialysis," says Dr. Suzanne Ildstad, a transplant surgeon and director of the Institute for Cellular Therapeutics at the University of Louisville, whose discovery of facilitator cells were the basis for this therapeutic platform. Ildstad, who has spent more than two decades searching for a better way, says, "This is something I have worked for my entire life."
The Louisville group uses a combination of chemo and radiation to replace the recipient's immune and blood forming cells with that of the donor. In contrast, the Stanford protocol involves harvesting the donor's blood stem cells and T-cells, which are the foot soldiers of the immune system that fight off infections and would normally orchestrate the rejection of the transplanted organ. Their transplant recipients undergo a milder form of "conditioning" that only radiates discrete parts of the body and selectively targets the recipient's T-cells, creating room for both sets of T-cells, a strategy these researchers believe has a better safety profile and less of a chance of rejection.
"We try to achieve immune tolerance by a true chimerism," says Dr. Samuel Strober, a professor of medicine for immunology and rheumatology at Stanford University and a leader of this research team. "The recipients immune system cells are maintained but mixed in the blood with that of the donor."
Studies suggest both approaches work. In a 2018 clinical trial conducted by Talaris Therapeutics, a Louisville-based biotech founded by Ildstad, 26 of 37 (70%) of the live donor kidney transplant recipients no longer need immunosuppressants. Last fall, Talaris began the final phase of clinical tests that will eventually encompass more than 120 such patients.
The Stanford group's cell-based immunotherapy, which is called MDR-101 and is sponsored by the South San Francisco biotech, Medeor Therapeutics, has had similar results in patients who received organs from live donors who were either well matched, such as one from siblings, meaning they were immunologically identical, or partially matched; Talaris uses unrelated donors where there is only a partial match.
In their 2020 clinical trial of 51 patients, 29 were fully matched and 22 were a partial match; 22 of the fully matched recipients didn't need antirejection drugs and ten of the partial matches were able to stop taking some of these medications without rejection. "With our fully matched, roughly 80% have been completely off drugs up to 14 years later," says Strober, "and reducing the number of drugs from three to one [in the partial matches] means you have far fewer side effects. The goal is to get them off of all drugs."
But these protocols are limited to a small number of patients—living donor kidney recipients. As a consequence, both teams are experimenting with ways to broaden their approach so they can use cadaver organs from deceased donors, with human tests planned in the coming year. Here's how that would work: after the other organs are removed from a deceased donor, stem cells are harvested from the donor's vertebrae in the spinal column and then frozen for storage.
"We do the transplant and give the patient a chance to recover and maintain them on drugs," says Ildstad. "Then we do the tolerance conditioning at a later stage."
If this strategy is successful, it would be a genuine game changer, and open the door to using these protocols for transplanting other cadaver organs, including the heart, lungs and liver. While the overall procedure is complex and costly, in the long run it's less expensive than repeated transplant surgeries, the cost of medications and hospitalizations for complications caused by the drugs, or thrice weekly dialysis treatments, says Ildstad.
And she adds, you can't put a price tag on the vast improvement in quality of life.
Tech-related injuries are becoming more common as many people depend on - and often develop addictions for - smart phones and computers.
In the 1990s, a mysterious virus spread throughout the Massachusetts Institute of Technology Artificial Intelligence Lab—or that’s what the scientists who worked there thought. More of them rubbed their aching forearms and massaged their cricked necks as new computers were introduced to the AI Lab on a floor-by-floor basis. They realized their musculoskeletal issues coincided with the arrival of these new computers—some of which were mounted high up on lab benches in awkward positions—and the hours spent typing on them.
Today, these injuries have become more common in a society awash with smart devices, sleek computers, and other gadgets. And we don’t just get hurt from typing on desktop computers; we’re massaging our sore wrists from hours of texting and Facetiming on phones, especially as they get bigger in size.
In 2007, the first iPhone measured 3.5-inches diagonally, a measurement known as the display size. That’s been nearly doubled by the newest iPhone 13 Pro, which has a 6.7-inch display. Other phones, too, like the Google Pixel 6 and the Samsung Galaxy S22, have bigger screens than their predecessors. Physical therapists and orthopedic surgeons have had to come up with names for a variety of new conditions: selfie elbow, tech neck, texting thumb. Orthopedic surgeon Sonya Sloan says she sees selfie elbow in younger kids and in women more often than men. She hears complaints related to technology once or twice a day.
The addictive quality of smartphones and social media means that people spend more time on their devices, which exacerbates injuries. According to Statista, 68 percent of those surveyed spent over three hours a day on their phone, and almost half spent five to six hours a day. Another report showed that people dedicate a third of their day to checking their phones, while the Media Effects Research Laboratory at Pennsylvania State University has found that bigger screens, ideal for entertainment purposes, immerse their users more than smaller screens. Oversized screens also provide easier navigation and more space for those with bigger hands or trouble seeing.
But others with conditions like arthritis can benefit from smaller phones. In March of 2016, Apple released the iPhone SE with a display size of 4.7 inches—an inch smaller than the iPhone 7, released that September. Apple has since come out with two more versions of the diminutive iPhone SE, one in 2020 and another in 2022.
These devices are now an inextricable part of our lives. So where does the burden of responsibility lie? Is it with consumers to adjust body positioning, get ergonomic workstations, and change habits to abate tech-related pain? Or should tech companies be held accountable?
Kavin Senapathy, a freelance science journalist, has the Google Pixel 6. She was drawn to the phone because Google marketed the Pixel 6’s camera as better at capturing different skin tones. But this phone boasts one of the largest display sizes on the market: 6.4 inches.
Senapathy was diagnosed with carpal and cubital tunnel syndromes in 2017 and fibromyalgia in 2019. She has had to create a curated ergonomic workplace setup, otherwise her wrists and hands get weak and tingly, and she’s had to adjust how she holds her phone to prevent pain flares.
Recently, Senapathy underwent an electromyography, or an EMG, in which doctors insert electrodes into muscles to measure their electrical activity. The electrical response of the muscles tells doctors whether the nerve cells and muscles are successfully communicating. Depending on her results, steroid shots and even surgery might be required. Senapathy wants to stick with her Pixel 6, but the pain she’s experiencing may push her to buy a smaller phone. Unfortunately, options for these modestly sized phones are more limited.
These devices are now an inextricable part of our lives. So where does the burden of responsibility lie? Is it with consumers like Senapathy to adjust body positioning, get ergonomic workstations, and change habits to abate tech-related pain? Or should tech companies be held accountable for creating addictive devices that lead to musculoskeletal injury?

Kavin Senapathy, a freelance journalist, bought the Google Pixel 6 because of its high-quality camera, but she’s had to adjust how she holds the oversized phone to prevent pain flares.
Kavin Senapathy
A one-size-fits-all mentality for smartphones will continue to lead to injuries because every user has different wants and needs. S. Shyam Sundar, the founder of Penn State’s lab on media effects and a communications professor, says the needs for mobility and portability conflict with the desire for greater visibility. “The best thing a company can do is offer different sizes,” he says.
Joanna Bryson, an AI ethics expert and professor at The Hertie School of Governance in Berlin, Germany, echoed these sentiments. “A lot of the lack of choice we see comes from the fact that the markets have consolidated so much,” she says. “We want to make sure there’s sufficient diversity [of products].”
Consumers can still maintain some control despite the ubiquity of tech. Sloan, the orthopedic surgeon, has to pester her son to change his body positioning when using his tablet. Our heads get heavier as they bend forward: at rest, they weigh 12 pounds, but bent 60 degrees, they weigh 60. “I have to tell him, ‘Raise your head, son!’” she says. It’s important, Sloan explains, to consider that growth and development will affect ligaments and bones in the neck, potentially making kids even more vulnerable to injuries from misusing gadgets. She recommends that parents limit their kids’ tech time to alleviate strain. She also suggested that tech companies implement a timer to remind us to change our body positioning.
In 2017, Nan-Wei Gong, a former contractor for Google, founded Figur8, which uses wearable trackers to measure muscle function and joint movement. It’s like physical therapy with biofeedback. “Each unique injury has a different biomarker,” says Gong. “With Figur8, you are comparing yourself to yourself.” This allows an individual to self-monitor for wear and tear and strengthen an injury in a way that’s efficient and designed for their body. Gong noticed that the work-from-home model during the COVID-19 pandemic created a new set of ergonomic problems that resulted in injuries. Figur8 provides real-time data for these injuries because “behavioral change requires feedback.”
Gong worked on a project called Jacquard while at Google. Textile experts weave conductive thread into their fabric, and the result is a patch of the fabric—like the cuff of a Levi’s jacket—that responds to commands on your smartphone. One swipe can call your partner or check the weather. It was designed with cyclists in mind who can’t easily check their phones, and it’s part of a growing movement in the tech industry to deliver creative, hands-free design. Gong thinks that engineers at large corporations like Google have accessibility in mind; it’s part of what drives their decisions for new products.
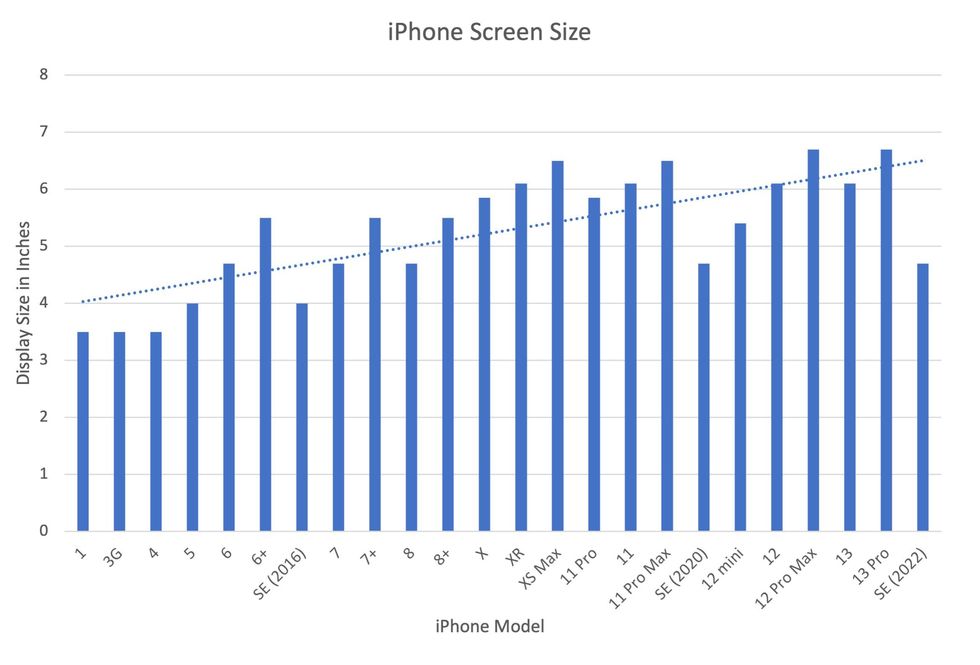
Display sizes of iPhones have become larger over time.
Sourced from Screenrant https://screenrant.com/iphone-apple-release-chronological-order-smartphone/ and Apple Tech Specs: https://www.apple.com/iphone-se/specs/
Back in Germany, Joanna Bryson reminds us that products like smartphones should adhere to best practices. These rules may be especially important for phones and other products with AI that are addictive. Disclosure, accountability, and regulation are important for AI, she says. “The correct balance will keep changing. But we have responsibilities and obligations to each other.” She was on an AI Ethics Council at Google, but the committee was disbanded after only one week due to issues with one of their members.
Bryson was upset about the Council’s dissolution but has faith that other regulatory bodies will prevail. OECD.AI, and international nonprofit, has drafted policies to regulate AI, which countries can sign and implement. “As of July 2021, 46 governments have adhered to the AI principles,” their website reads.
Sundar, the media effects professor, also directs Penn State’s Center for Socially Responsible AI. He says that inclusivity is a crucial aspect of social responsibility and how devices using AI are designed. “We have to go beyond first designing technologies and then making them accessible,” he says. “Instead, we should be considering the issues potentially faced by all different kinds of users before even designing them.”
Entomologist Jessica Ware is using new technologies to identify insect species in a changing climate. She shares her suggestions for how we can live harmoniously with creeper crawlers everywhere.
Jessica Ware is obsessed with bugs.
My guest today is a leading researcher on insects, the president of the Entomological Society of America and a curator at the American Museum of Natural History. Learn more about her here.
You may not think that insects and human health go hand-in-hand, but as Jessica makes clear, they’re closely related. A lot of people care about their health, and the health of other creatures on the planet, and the health of the planet itself, but researchers like Jessica are studying another thing we should be focusing on even more: how these seemingly separate areas are deeply entwined. (This is the theme of an upcoming event hosted by Leaps.org and the Aspen Institute.)
Listen to the Episode
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google

Entomologist Jessica Ware
D. Finnin / AMNH
Maybe it feels like a core human instinct to demonize bugs as gross. We seem to try to eradicate them in every way possible, whether that’s with poison, or getting out our blood thirst by stomping them whenever they creep and crawl into sight.
But where did our fear of bugs really come from? Jessica makes a compelling case that a lot of it is cultural, rather than in-born, and we should be following the lead of other cultures that have learned to live with and appreciate bugs.
The truth is that a healthy planet depends on insects. You may feel stung by that news if you hate bugs. Reality bites.
Jessica and I talk about whether learning to live with insects should include eating them and gene editing them so they don’t transmit viruses. She also tells me about her important research into using genomic tools to track bugs in the wild to figure out why and how we’ve lost 50 percent of the insect population since 1970 according to some estimates – bad news because the ecosystems that make up the planet heavily depend on insects. Jessica is leading the way to better understand what’s causing these declines in order to start reversing these trends to save the insects and to save ourselves.

