One Day, There Might Be a Drug for a Broken Heart

A sad woman peering through sunlit blinds.
For Tony Y., 37, healing from heartbreak is slow and incomplete. Each of several exes is associated with a cluster of sore memories. Although he loves the Blue Ridge Mountains, he can't visit because they remind him of a romantic holiday years ago.
If a new drug made rejections less painful, one expert argues, it could relieve or even prevent major depression.
Like some 30 to 40 percent of depressed patients, Tony hasn't had success with current anti-depressants. One day, psychiatrists may be able to offer him a new kind of opioid, an anti-depressant for people suffering from the cruel pain of rejection.
A Surprising Discovery
As we move through life, rejections -- bullying in school, romantic breakups, and divorces -- are powerful triggers to depressive episodes, observes David Hsu, a neuroscientist at Stony Brook University School of Medicine in Long Island, New York. If a new drug made them less painful, he argues, it could relieve or even prevent major depression.
Our bodies naturally produce opioids to soothe physical pain, and opioid drugs like morphine and oxycodone work by plugging into the same receptors in our brains. The same natural opioids may also respond to emotional hurts, and painkillers can dramatically affect mood. Today's epidemic of opioid abuse raises the question: How many lives might have been saved if we had a safe, non-addictive option for medicating emotional pain?
Already one anti-depressant, tianeptine, locks into the mu opioid receptor, the target of morphine and oxycodone. Scientists knew that tianeptine, prescribed in some countries in Europe, Asia, and Latin America, acted differently than the most common anti-depressants in use today, which affect the levels of other brain chemicals, serotonin and norepinephrine. But the discovery in 2014 that tianeptine tapped the mu receptor was a "huge surprise," says co-author Jonathan Javitch, chief of the Division of Molecular Therapeutics at Columbia University.
The news arrived when scientists' basic understanding of depression is in flux; viewed biologically, it may cover several disorders. One of them could hinge on opioids. It's possible that some people release fewer opioids naturally or that the receptors for it are less effective.
Javitch has launched a startup, Kures, to make tianeptine more effective and convenient and to find other opioid-modulators. That may seem quixotic in the midst of an opioid epidemic, but tianeptine doesn't create dependency in low, prescription doses and has been used safely around the world for decades. To identify likely patients, cofounder Andrew Kruegel is looking for ways to "segment the depressed population by measures that have to do with opioid release," he says.
Is Emotional Pain Actually "Pain"?
No one imagines that the pain from rejection or loss is the same as pain from a broken leg. Physical pain is two perceptions—a sensory perception and an "affective" one, which makes pain unpleasant.
Exploration of an overlap between physical and what research psychologists call "social pain" has heated up since the mid-2000s.
The sensory perception, processed by regions of the brain called the primary and secondary somatosensory cortices and the posterior insula, tells us whether the pain is in your arm or your leg, how strong it is and whether it is a sting, ache, or has some other quality. The affective perception, in another part of the brain called the dorsal anterior cingulate cortex and the anterior insula, tells us that we want the pain to stop, fast! When people with lesions in the latter areas experience a stimulus that ordinarily would be painful, they don't mind it.
Science now suggests that emotional pain arises in the affective brain circuits. Exploration of an overlap between physical and what research psychologists call "social pain" has heated up since the mid-2000s. Animal evidence goes back to the 1970s: babies separated from their mothers showed less distress when given morphine, and more if dosed with naloxone, the opioid antagonist.
Parents, of course, face the question of whether Baby feels alone or wet whenever she howls. And the answer is: both hurt. Being abandoned is the ultimate threat in our early life, and it makes sense that a brain system to monitor social threats would piggyback upon an existing system for pain. Piggybacking is a feature of evolution. An ancestor who felt "hurt" when threatened by rejection might learn adaptive behavior: to cooperate or run.
In 2010, a large multi-university team led by Nathan DeWall at the University of Kentucky, reported that acetaminophen (Tylenol) reduced social pain. Undergraduates took 500 mg of acetaminophen upon awakening and at bedtime every day for three weeks and reported nightly about their day using a previously-tested "Hurt Feelings Scale," rating how strongly they agreed with questions like, "Today, being teased hurt my feelings."
Over the weeks, their reports of hurt feelings steadily declined, while remaining flat in a control group that took placebos. In a second experiment, the research group showed that, compared to controls, people who had taken acetaminophen for three weeks showed less brain activity in the affective brain circuits while they experienced rejection during a virtual ball-tossing game. Later, Hsu's brain scan research supported the idea that rejection triggers the mu opioid receptor system, which normally provides pain-dampening opioids.
More evidence comes from nonhuman primates with lesions in the affective circuits: They cry less when separated from caregivers or social groups.
Heartbreak seems to lie in those regions: women with major depression are more hurt by romantic rejection than normal controls are and show more activity in those areas in brain scans, Hsu found. Also, factors that make us more vulnerable to rejection -- like low self-esteem -- are linked to more activity in the key areas, studies show.
The trait "high rejection sensitivity" increases your risk of depression more than "global neuroticism" does, Hsu observes, and predicts a poor recovery from depression. Pain sensitivity is another clue: People with a gene linked to it seem to be more hurt by social exclusion. Once you're depressed, you become more rejection-sensitive and prone to pain—a classic bad feedback loop.
"Ideally, we'd have biomarkers to distinguish when loss becomes complicated grief and then depression, and we might prevent the transition with a drug."
Helen Mayberg, a neurologist renowned for her study of brain circuits in depression, sees, as Hsu does, the possibility of preventing depressions. "Nobody would suggest we treat routine bad social pain with drugs. But it is true that in susceptible people, losing a partner, for example, can lead to a full-blown depression," says Mayberg, who is the founding director of The Center for Advanced Circuit Therapeutics at Mount Sinai's Icahn School of Medicine in New York City. "Ideally, we'd have biomarkers to distinguish when loss becomes complicated grief and then depression, and we might prevent the transition with a drug. It would be like taking medication when you feel the warning symptoms of a headache to prevent a full-blown migraine."
A Way Out of the Opioid Crisis?
The exploration of social pain should lead us to a deeper understanding of pain, beyond the sharp distinctions between "physical" and "psychological." Finding our way out of the current crisis may require that deeper understanding. About half of the people with opioid prescriptions have mental health disorders. "I expect there are a lot of people using street opioids—heroin or prescriptions purchased from others--to self-medicate psychological pain," Kreugel says.
What we may need, he suggests, is "a new paradigm for using opioids in psychiatry: low, sub-analgesic, sub-euphoric dosing." But so far it hasn't been easy. Investors don't flock to fund psychiatric drugs and in 2018, the word opioid is poison.
As for Tony Y., he's struggled for three years to recover from his most serious relationship. "Driving around highways looking at exit signs toward places we visited together sometimes fills me with unbearable anguish," he admits. "And because we used to do so much bird watching together, sometimes a mere glimpse of a random bird sets me off." He perks up at the idea of a heartbreak drug. "If the side effects didn't seem bad, I would consider it, absolutely."
DNA- and RNA-based electronic implants may revolutionize healthcare
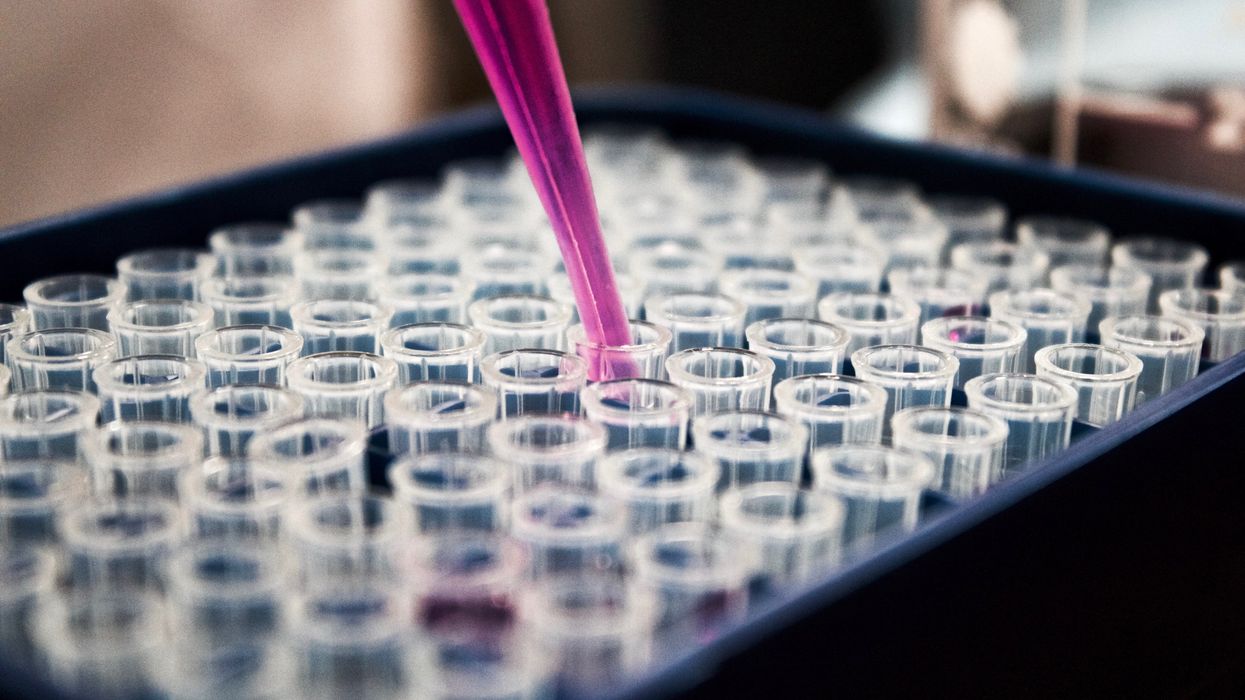
The test tubes contain tiny DNA/enzyme-based circuits, which comprise TRUMPET, a new type of electronic device, smaller than a cell.
Implantable electronic devices can significantly improve patients’ quality of life. A pacemaker can encourage the heart to beat more regularly. A neural implant, usually placed at the back of the skull, can help brain function and encourage higher neural activity. Current research on neural implants finds them helpful to patients with Parkinson’s disease, vision loss, hearing loss, and other nerve damage problems. Several of these implants, such as Elon Musk’s Neuralink, have already been approved by the FDA for human use.
Yet, pacemakers, neural implants, and other such electronic devices are not without problems. They require constant electricity, limited through batteries that need replacements. They also cause scarring. “The problem with doing this with electronics is that scar tissue forms,” explains Kate Adamala, an assistant professor of cell biology at the University of Minnesota Twin Cities. “Anytime you have something hard interacting with something soft [like muscle, skin, or tissue], the soft thing will scar. That's why there are no long-term neural implants right now.” To overcome these challenges, scientists are turning to biocomputing processes that use organic materials like DNA and RNA. Other promised benefits include “diagnostics and possibly therapeutic action, operating as nanorobots in living organisms,” writes Evgeny Katz, a professor of bioelectronics at Clarkson University, in his book DNA- And RNA-Based Computing Systems.
While a computer gives these inputs in binary code or "bits," such as a 0 or 1, biocomputing uses DNA strands as inputs, whether double or single-stranded, and often uses fluorescent RNA as an output.
Adamala’s research focuses on developing such biocomputing systems using DNA, RNA, proteins, and lipids. Using these molecules in the biocomputing systems allows the latter to be biocompatible with the human body, resulting in a natural healing process. In a recent Nature Communications study, Adamala and her team created a new biocomputing platform called TRUMPET (Transcriptional RNA Universal Multi-Purpose GatE PlaTform) which acts like a DNA-powered computer chip. “These biological systems can heal if you design them correctly,” adds Adamala. “So you can imagine a computer that will eventually heal itself.”
The basics of biocomputing
Biocomputing and regular computing have many similarities. Like regular computing, biocomputing works by running information through a series of gates, usually logic gates. A logic gate works as a fork in the road for an electronic circuit. The input will travel one way or another, giving two different outputs. An example logic gate is the AND gate, which has two inputs (A and B) and two different results. If both A and B are 1, the AND gate output will be 1. If only A is 1 and B is 0, the output will be 0 and vice versa. If both A and B are 0, the result will be 0. While a computer gives these inputs in binary code or "bits," such as a 0 or 1, biocomputing uses DNA strands as inputs, whether double or single-stranded, and often uses fluorescent RNA as an output. In this case, the DNA enters the logic gate as a single or double strand.
If the DNA is double-stranded, the system “digests” the DNA or destroys it, which results in non-fluorescence or “0” output. Conversely, if the DNA is single-stranded, it won’t be digested and instead will be copied by several enzymes in the biocomputing system, resulting in fluorescent RNA or a “1” output. And the output for this type of binary system can be expanded beyond fluorescence or not. For example, a “1” output might be the production of the enzyme insulin, while a “0” may be that no insulin is produced. “This kind of synergy between biology and computation is the essence of biocomputing,” says Stephanie Forrest, a professor and the director of the Biodesign Center for Biocomputing, Security and Society at Arizona State University.
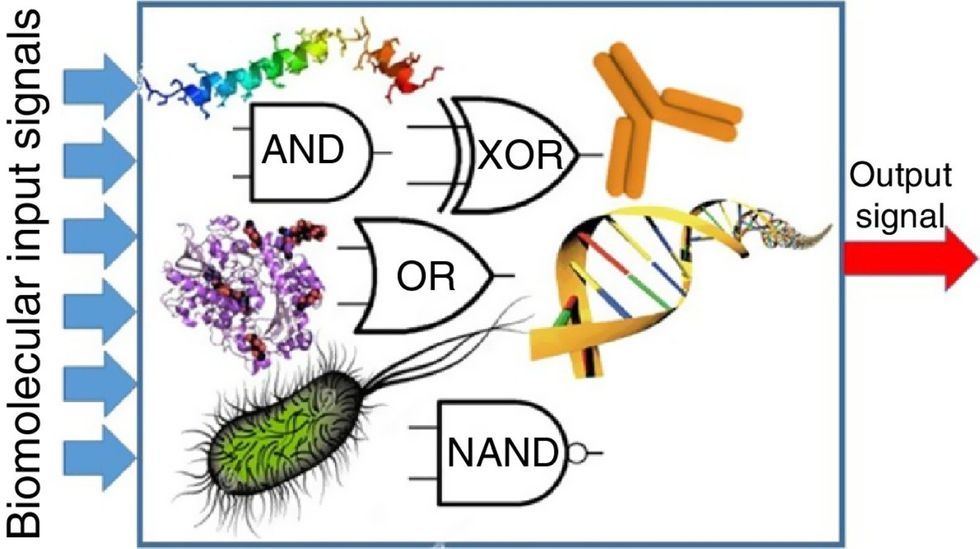
Biocomputing circles are made of DNA, RNA, proteins and even bacteria.
Evgeny Katz
The TRUMPET’s promise
Depending on whether the biocomputing system is placed directly inside a cell within the human body, or run in a test-tube, different environmental factors play a role. When an output is produced inside a cell, the cell's natural processes can amplify this output (for example, a specific protein or DNA strand), creating a solid signal. However, these cells can also be very leaky. “You want the cells to do the thing you ask them to do before they finish whatever their businesses, which is to grow, replicate, metabolize,” Adamala explains. “However, often the gate may be triggered without the right inputs, creating a false positive signal. So that's why natural logic gates are often leaky." While biocomputing outside a cell in a test tube can allow for tighter control over the logic gates, the outputs or signals cannot be amplified by a cell and are less potent.
TRUMPET, which is smaller than a cell, taps into both cellular and non-cellular biocomputing benefits. “At its core, it is a nonliving logic gate system,” Adamala states, “It's a DNA-based logic gate system. But because we use enzymes, and the readout is enzymatic [where an enzyme replicates the fluorescent RNA], we end up with signal amplification." This readout means that the output from the TRUMPET system, a fluorescent RNA strand, can be replicated by nearby enzymes in the platform, making the light signal stronger. "So it combines the best of both worlds,” Adamala adds.
These organic-based systems could detect cancer cells or low insulin levels inside a patient’s body.
The TRUMPET biocomputing process is relatively straightforward. “If the DNA [input] shows up as single-stranded, it will not be digested [by the logic gate], and you get this nice fluorescent output as the RNA is made from the single-stranded DNA, and that's a 1,” Adamala explains. "And if the DNA input is double-stranded, it gets digested by the enzymes in the logic gate, and there is no RNA created from the DNA, so there is no fluorescence, and the output is 0." On the story's leading image above, if the tube is "lit" with a purple color, that is a binary 1 signal for computing. If it's "off" it is a 0.
While still in research, TRUMPET and other biocomputing systems promise significant benefits to personalized healthcare and medicine. These organic-based systems could detect cancer cells or low insulin levels inside a patient’s body. The study’s lead author and graduate student Judee Sharon is already beginning to research TRUMPET's ability for earlier cancer diagnoses. Because the inputs for TRUMPET are single or double-stranded DNA, any mutated or cancerous DNA could theoretically be detected from the platform through the biocomputing process. Theoretically, devices like TRUMPET could be used to detect cancer and other diseases earlier.
Adamala sees TRUMPET not only as a detection system but also as a potential cancer drug delivery system. “Ideally, you would like the drug only to turn on when it senses the presence of a cancer cell. And that's how we use the logic gates, which work in response to inputs like cancerous DNA. Then the output can be the production of a small molecule or the release of a small molecule that can then go and kill what needs killing, in this case, a cancer cell. So we would like to develop applications that use this technology to control the logic gate response of a drug’s delivery to a cell.”
Although platforms like TRUMPET are making progress, a lot more work must be done before they can be used commercially. “The process of translating mechanisms and architecture from biology to computing and vice versa is still an art rather than a science,” says Forrest. “It requires deep computer science and biology knowledge,” she adds. “Some people have compared interdisciplinary science to fusion restaurants—not all combinations are successful, but when they are, the results are remarkable.”
Crickets are low on fat, high on protein, and can be farmed sustainably. They are also crunchy.
In today’s podcast episode, Leaps.org Deputy Editor Lina Zeldovich speaks about the health and ecological benefits of farming crickets for human consumption with Bicky Nguyen, who joins Lina from Vietnam. Bicky and her business partner Nam Dang operate an insect farm named CricketOne. Motivated by the idea of sustainable and healthy protein production, they started their unconventional endeavor a few years ago, despite numerous naysayers who didn’t believe that humans would ever consider munching on bugs.
Yet, making creepy crawlers part of our diet offers many health and planetary advantages. Food production needs to match the rise in global population, estimated to reach 10 billion by 2050. One challenge is that some of our current practices are inefficient, polluting and wasteful. According to nonprofit EarthSave.org, it takes 2,500 gallons of water, 12 pounds of grain, 35 pounds of topsoil and the energy equivalent of one gallon of gasoline to produce one pound of feedlot beef, although exact statistics vary between sources.
Meanwhile, insects are easy to grow, high on protein and low on fat. When roasted with salt, they make crunchy snacks. When chopped up, they transform into delicious pâtes, says Bicky, who invents her own cricket recipes and serves them at industry and public events. Maybe that’s why some research predicts that edible insects market may grow to almost $10 billion by 2030. Tune in for a delectable chat on this alternative and sustainable protein.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
Further reading:
More info on Bicky Nguyen
https://yseali.fulbright.edu.vn/en/faculty/bicky-n...
The environmental footprint of beef production
https://www.earthsave.org/environment.htm
https://www.watercalculator.org/news/articles/beef-king-big-water-footprints/
https://www.frontiersin.org/articles/10.3389/fsufs.2019.00005/full
https://ourworldindata.org/carbon-footprint-food-methane
Insect farming as a source of sustainable protein
https://www.insectgourmet.com/insect-farming-growing-bugs-for-protein/
https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/insect-farming
Cricket flour is taking the world by storm
https://www.cricketflours.com/
https://talk-commerce.com/blog/what-brands-use-cricket-flour-and-why/

Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.