After spaceflight record, NASA looks to protect astronauts on even longer trips

NASA astronaut Frank Rubio floats by the International Space Station’s “window to the world.” Yesterday, he returned from the longest single spaceflight by a U.S. astronaut on record - over one year. Exploring deep space will require even longer missions.
At T-minus six seconds, the main engines of the Atlantis Space Shuttle ignited, rattling its capsule “like a skyscraper in an earthquake,” according to astronaut Tom Jones, describing the 1988 launch. As the rocket lifted off and accelerated to three times the force of Earth's gravity, “It felt as if two of my friends were standing on my chest and wouldn’t get off.” But when Atlantis reached orbit, the main engines cut off, and the astronauts were suddenly weightless.
Since 1961, NASA has sent hundreds of astronauts into space while working to making their voyages safer and smoother. Yet, challenges remain. Weightlessness may look amusing when watched from Earth, but it has myriad effects on cognition, movement and other functions. When missions to space stretch to six months or longer, microgravity can impact astronauts’ health and performance, making it more difficult to operate their spacecraft.
Yesterday, NASA astronaut Frank Rubio returned to Earth after over one year, the longest single spaceflight for a U.S. astronaut. But this is just the start; longer and more complex missions into deep space loom ahead, from returning to the moon in 2025 to eventually sending humans to Mars. To ensure that these missions succeed, NASA is increasing efforts to study the biological effects and prevent harm.
The dangers of microgravity are real
A NASA report published in 2016 details a long list of incidents and near-misses caused – at least partly – by space-induced changes in astronauts’ vision and coordination. These issues make it harder to move with precision and to judge distance and velocity.
According to the report, in 1997, a resupply ship collided with the Mir space station, possibly because a crew member bumped into the commander during the final docking maneuver. This mishap caused significant damage to the space station.
Returns to Earth suffered from problems, too. The same report notes that touchdown speeds during the first 100 space shuttle landings were “outside acceptable limits. The fastest landing on record – 224 knots (258 miles) per hour – was linked to the commander’s momentary spatial disorientation.” Earlier, each of the six Apollo crews that landed on the moon had difficulty recognizing moon landmarks and estimating distances. For example, Apollo 15 landed in an unplanned area, ultimately straddling the rim of a five-foot deep crater on the moon, harming one of its engines.
Spaceflight causes unique stresses on astronauts’ brains and central nervous systems. NASA is working to reduce these harmful effects.
NASA
Space messes up your brain
In space, astronauts face the challenges of microgravity, ionizing radiation, social isolation, high workloads, altered circadian rhythms, monotony, confined living quarters and a high-risk environment. Among these issues, microgravity is one of the most consequential in terms of physiological changes. It changes the brain’s structure and its functioning, which can hurt astronauts’ performance.
The brain shifts upwards within the skull, displacing the cerebrospinal fluid, which reduces the brain’s cushioning. Essentially, the brain becomes crowded inside the skull like a pair of too-tight shoes.
That’s partly because of how being in space alters blood flow. On Earth, gravity pulls our blood and other internal fluids toward our feet, but our circulatory valves ensure that the fluids are evenly distributed throughout the body. In space, there’s not enough gravity to pull the fluids down, and they shift up, says Rachael D. Seidler, a physiologist specializing in spaceflight at the University of Florida and principal investigator on many space-related studies. The head swells and legs appear thinner, causing what astronauts call “puffy face chicken legs.”
“The brain changes at the structural and functional level,” says Steven Jillings, equilibrium and aerospace researcher at the University of Antwerp in Belgium. “The brain shifts upwards within the skull,” displacing the cerebrospinal fluid, which reduces the brain’s cushioning. Essentially, the brain becomes crowded inside the skull like a pair of too-tight shoes. Some of the displaced cerebrospinal fluid goes into cavities within the brain, called ventricles, enlarging them. “The remaining fluids pool near the chest and heart,” explains Jillings. After 12 consecutive months in space, one astronaut had a ventricle that was 25 percent larger than before the mission.
Some changes reverse themselves while others persist for a while. An example of a longer-lasting problem is spaceflight-induced neuro-ocular syndrome, which results in near-sightedness and pressure inside the skull. A study of approximately 300 astronauts shows near-sightedness affects about 60 percent of astronauts after long missions on the International Space Station (ISS) and more than 25 percent after spaceflights of only a few weeks.
Another long-term change could be the decreased ability of cerebrospinal fluid to clear waste products from the brain, Seidler says. That’s because compressing the brain also compresses its waste-removing glymphatic pathways, resulting in inflammation, vulnerability to injuries and worsening its overall health.
The effects of long space missions were best demonstrated on astronaut twins Scott and Mark Kelly. This NASA Twins Study showed multiple, perhaps permanent, changes in Scott after his 340-day mission aboard the ISS, compared to Mark, who remained on Earth. The differences included declines in Scott’s speed, accuracy and cognitive abilities that persisted longer than six months after returning to Earth in March 2016.
By the end of 2020, Scott’s cognitive abilities improved, but structural and physiological changes to his eyes still remained, he said in a BBC interview.
“It seems clear that the upward shift of the brain and compression of the surrounding tissues with ventricular expansion might not be a good thing,” Seidler says. “But, at this point, the long-term consequences to brain health and human performance are not really known.”

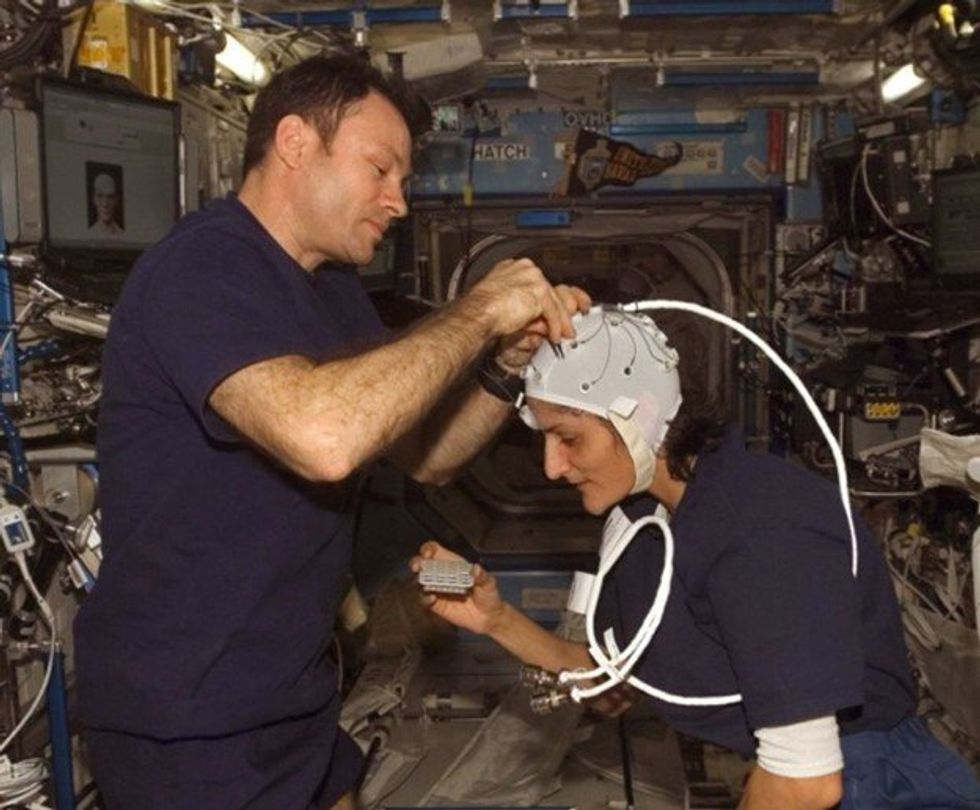
NASA astronaut Kate Rubins conducts a session for the Neuromapping investigation.
NASA
Staying sharp in space
To investigate how prolonged space travel affects the brain, NASA launched a new initiative called the Complement of Integrated Protocols for Human Exploration Research (CIPHER). “CIPHER investigates how long-duration spaceflight affects both brain structure and function,” says neurobehavioral scientist Mathias Basner at the University of Pennsylvania, a principal investigator for several NASA studies. “Through it, we can find out how the brain adapts to the spaceflight environment and how certain brain regions (behave) differently after – relative to before – the mission.”
To do this, he says, “Astronauts will perform NASA’s cognition test battery before, during and after six- to 12-month missions, and will also perform the same test battery in an MRI scanner before and after the mission. We have to make sure we better understand the functional consequences of spaceflight on the human brain before we can send humans safely to the moon and, especially, to Mars.”
As we go deeper into space, astronauts cognitive and physical functions will be even more important. “A trip to Mars will take about one year…and will introduce long communication delays,” Seidler says. “If you are on that mission and have a problem, it may take eight to 10 minutes for your message to reach mission control, and another eight to 10 minutes for the response to get back to you.” In an emergency situation, that may be too late for the response to matter.
“On a mission to Mars, astronauts will be exposed to stressors for unprecedented amounts of time,” Basner says. To counter them, NASA is considering the continuous use of artificial gravity during the journey, and Seidler is studying whether artificial gravity can reduce the harmful effects of microgravity. Some scientists are looking at precision brain stimulation as a way to improve memory and reduce anxiety due to prolonged exposure to radiation in space.
Other scientists are exploring how to protect neural stem cells (which create brain cells) from radiation damage, developing drugs to repair damaged brain cells and protect cells from radiation.
To boldly go where no astronauts have gone before, they must have optimal reflexes, vision and decision-making. In the era of deep space exploration, the brain—without a doubt—is the final frontier.
Additionally, NASA is scrutinizing each aspect of the mission, including astronaut exercise, nutrition and intellectual engagement. “We need to give astronauts meaningful work. We need to stimulate their sensory, cognitive and other systems appropriately,” Basner says, especially given their extreme confinement and isolation. The scientific experiments performed on the ISS – like studying how microgravity affects the ability of tissue to regenerate is a good example.
“We need to keep them engaged socially, too,” he continues. The ISS crew, for example, regularly broadcasts from space and answers prerecorded questions from students on Earth, and can engage with social media in real time. And, despite tight quarters, NASA is ensuring the crew capsule and living quarters on the moon or Mars include private space, which is critical for good mental health.
Exploring deep space builds on a foundation that began when astronauts first left the planet. With each mission, scientists learn more about spaceflight effects on astronauts’ bodies. NASA will be using these lessons to succeed with its plans to build science stations on the moon and, eventually, Mars.
“Through internally and externally led research, investigations implemented in space and in spaceflight simulations on Earth, we are striving to reduce the likelihood and potential impacts of neurostructural changes in future, extended spaceflight,” summarizes NASA scientist Alexandra Whitmire. To boldly go where no astronauts have gone before, they must have optimal reflexes, vision and decision-making. In the era of deep space exploration, the brain—without a doubt—is the final frontier.
With this new technology, hospitals and pharmacies could make vaccines and medicines onsite
New research focuses on methods that could change medicine-making worldwide. The scientists propose bursting cells open, removing their DNA and using the cellular gears inside to make therapies.
Most modern biopharmaceutical medicines are produced by workhorse cells—typically bacterial but sometimes mammalian. The cells receive the synthesizing instructions on a snippet of a genetic code, which they incorporate into their DNA. The cellular machinery—ribosomes, RNAs, polymerases, and other compounds—read and use these instructions to build the medicinal molecules, which are harvested and administered to patients.
Although a staple of modern pharma, this process is complex and expensive. One must first insert the DNA instructions into the cells, which they may or may not uptake. One then must grow the cells, keeping them alive and well, so that they produce the required therapeutics, which then must be isolated and purified. To make this at scale requires massive bioreactors and big factories from where the drugs are distributed—and may take a while to arrive where they’re needed. “The pandemic showed us that this method is slow and cumbersome,” says Govind Rao, professor of biochemical engineering who directs the Center for Advanced Sensor Technology at the University of Maryland, Baltimore County (UMBC). “We need better methods that can work faster and can work locally where an outbreak is happening.”
Rao and his team of collaborators, which spans multiple research institutions, believe they have a better approach that may change medicine-making worldwide. They suggest forgoing the concept of using living cells as medicine-producers. Instead, they propose breaking the cells and using the remaining cellular gears for assembling the therapeutic compounds. Instead of inserting the DNA into living cells, the team burst them open, and removed their DNA altogether. Yet, the residual molecular machinery of ribosomes, polymerases and other cogwheels still functioned the way it would in a cell. “Now if you drop your DNA drug-making instructions into that soup, this machinery starts making what you need,” Rao explains. “And because you're no longer worrying about living cells, it becomes much simpler and more efficient.” The collaborators detail their cell-free protein synthesis or CFPS method in their recent paper published in preprint BioAxiv.
While CFPS does not use living cells, it still needs the basic building blocks to assemble proteins from—such as amino acids, nucleotides and certain types of enzymes. These are regularly added into this “soup” to keep the molecular factory chugging. “We just mix everything in as a batch and we let it integrate,” says James Robert Swartz, professor of chemical engineering and bioengineering at Stanford University and co-author of the paper. “And we make sure that we provide enough oxygen.” Rao likens the process to making milk from milk powder.
For a variety of reasons—from the field’s general inertia to regulatory approval hurdles—the method hasn’t become mainstream. The pandemic rekindled interest in medicines that can be made quickly and easily, so it drew more attention to the technology.
The idea of a cell-free protein synthesis is older than one might think. Swartz first experimented with it around 1997, when he was a chemical engineer at Genentech. While working on engineering bacteria to make pharmaceuticals, he discovered that there was a limit to what E. coli cells, the workhorse darling of pharma, could do. For example, it couldn’t grow and properly fold some complex proteins. “We tried many genetic engineering approaches, many fermentation, development, and environmental control approaches,” Swartz recalls—to no avail.
“The organism had its own agenda,” he quips. “And because everything was happening within the organism, we just couldn't really change those conditions very easily. Some of them we couldn’t change at all—we didn’t have control.”
It was out of frustration with the defiant bacteria that a new idea took hold. Could the cells be opened instead, so that the protein-forming reactions could be influenced more easily? “Obviously, we’d lose the ability for them to reproduce,” Swartz says. But that also meant that they no longer needed to keep the cells alive and could focus on making the specific reactions happen. “We could take the catalysts, the enzymes, and the more complex catalysts and activate them, make them work together, much as they would in a living cell, but the way we wanted.”
In 1998, Swartz joined Stanford, and began perfecting the biochemistry of the cell-free method, identifying the reactions he wanted to foster and stopping those he didn’t want. He managed to make the idea work, but for a variety of reasons—from the field’s general inertia to regulatory approval hurdles—the method hasn’t become mainstream. The pandemic rekindled interest in medicines that can be made quickly and easily, so it drew more attention to the technology. For their BioArxiv paper, the team tested the method by growing a specific antiviral protein called griffithsin.
First identified by Barry O’Keefe at National Cancer Institute over a decade ago, griffithsin is an antiviral known to interfere with many viruses’ ability to enter cells—including HIV, SARS, SARS-CoV-2, MERS and others. Originally isolated from the red algae Griffithsia, it works differently from antibodies and antibody cocktails.
Most antiviral medicines tend to target the specific receptors that viruses use to gain entry to the cells they infect. For example, SARS-CoV-2 uses the infamous spike protein to latch onto the ACE2 receptor of mammalian cells. The antibodies or other antiviral molecules stick to the spike protein, shutting off its ability to cling onto the ACE2 receptors. Unfortunately, the spike proteins mutate very often, so the medicines lose their potency. On the contrary, griffithsin has the ability to cling to the different parts of viral shells called capsids—namely to the molecules of mannose, a type of sugar. That extra stuff, glued all around the capsid like dead weight, makes it impossible for the virus to squeeze into the cell.
“Every time we have a vaccine or an antibody against a specific SARS-CoV-2 strain, that strain then mutates and so you lose efficacy,” Rao explains. “But griffithsin molecules glom onto the viral capsid, so the capsid essentially becomes a sticky mess and can’t enter the cell.” Mannose molecules also don’t mutate as easily as viruses’ receptors, so griffithsin-based antivirals do not have to be constantly updated. And because mannose molecules are found on many viruses’ capsids, it makes griffithsin “a universal neutralizer,” Rao explains.
“When griffithsin was discovered, we recognized that it held a lot of promise as a potential antiviral agent,” O’Keefe says. In 2010, he published a paper about griffithsin efficacy in neutralizing viruses of the corona family—after the first SARS outbreak in the early 2000s, the scientific community was interested in such antivirals. Yet, griffithsin is still not available as an off-the-shelf product. So during the Covid pandemic, the team experimented with synthesizing griffithsin using the cell-free production method. They were able to generate potent griffithsin in less than 24 hours without having to grow living cells.
The antiviral protein isn't the only type of medicine that can be made cell-free. The proteins needed for vaccine production could also be made the same way. “Such portable, on-demand drug manufacturing platforms can produce antiviral proteins within hours, making them ideal for combating future pandemics,” Rao says. “We would be able to stop the pandemic before it spreads.”
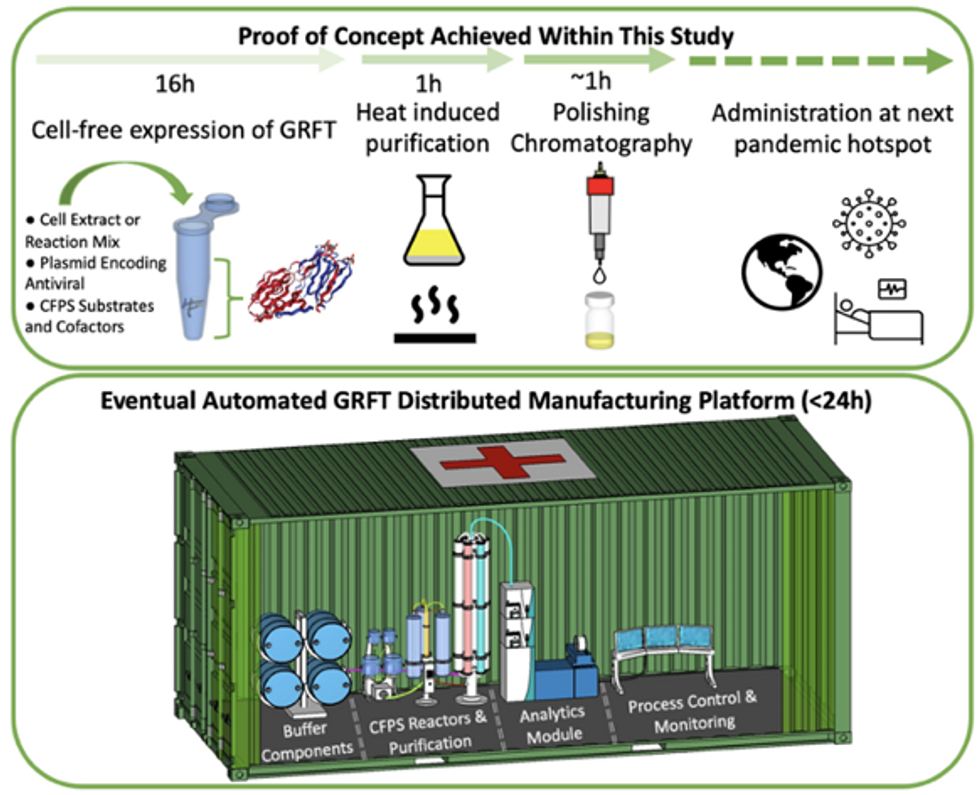
Top: Describes the process used in the study. Bottom: Describes how the new medicines and vaccines could be made at the site of a future viral outbreak.
Image courtesy of Rao and team, sourced from An approach to rapid distributed manufacturing of broad spectrumanti-viral griffithsin using cell-free systems to mitigate pandemics.
Rao’s idea is to perfect the technology to the point that any hospital or pharmacy can load up the media containing molecular factories, mix up the required amino acids, nucleotides and enzymes, and harvest the meds within hours. That will allow making medicines onsite and on demand. “That would be a self-contained production unit, so that you could just ship the production wherever the pandemic is breaking out,” says Swartz.
These units and the meds they produce, will, of course, have to undergo rigorous testing. “The biggest hurdles will be validating these against conventional technology,” Rao says. The biotech industry is risk-averse and prefers the familiar methods. But if this approach works, it may go beyond emergency situations and revolutionize the medicine-making paradigm even outside hospitals and pharmacies. Rao hopes that someday the method might become so mainstream that people may be able to buy and operate such reactors at home. “You can imagine a diabetic patient making insulin that way, or some other drugs,” Rao says. It would work not unlike making baby formula from the mere white powder. Just add water—and some oxygen, too.
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.
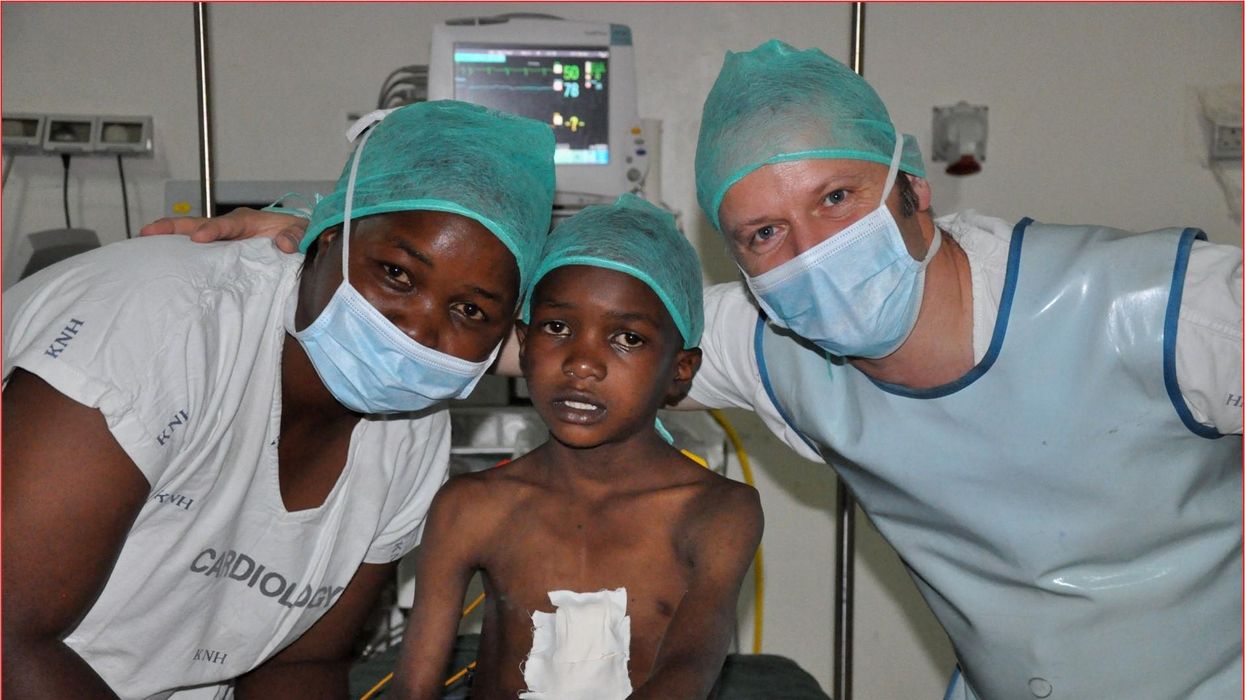
These doctors have a heart for recycling
In the U.S. and Europe, it is illegal to reuse pacemakers and other implants. Therefore, cardiologists export them to the global South where they save the lives of people of all ages.
This is part 3 of a three part series on a new generation of doctors leading the charge to make the health care industry more sustainable - for the benefit of their patients and the planet. Read part 1 here and part 2 here.
One could say that over 400 people owe their life to the fact that Carsten Israel fell in love. Twenty years ago, as a young doctor in Frankfurt, Germany, he began to court an au pair from Kenya, Elisabeth, his now-wife of 13 years with whom he has three children. When the couple started visiting her parents in Kenya, Israel wanted to check out the local hospitals, “just out of professional curiosity,“ says the cardiologist, who is currently the head doctor at the Clinic for Interior Medicine in Bielefeld. “I was completely shocked.“
Often he observed there were no doctors in the E.R.s, and hte nurses could render only basic first aid. “When somebody fell into a coma, they fell into a coma,“ Israel remembers. “There weren’t even any defibrillators to restart a patient’s heart,” while defibrillators are standard equipment in most clinics in the U.S. and Europe as lifesaving devices. When Israel finally visited the largest and most modern hospital in Nairobi, he found it better equipped but he learned that its services were only available to patients who could afford them. The cardiologist there had a drawer full of petitions from patients with heart ailments who couldn’t afford lifesaving surgery. Even two decades ago, a pacemaker cost $5,000 in Kenya, which made it unaffordable for most Kenyans who earn an average of $600 per month.
Since 2003, Israel and a team of two doctors and two nurses visit Kenya and Zambia once or twice a year to implant German pacemakers for free. Notably, the pacemakers and defibrillators Israel exports to Africa would end up in the landfill in Germany. Clinics have to pay for specialized services to dispose of this medical equipment. “In Germany, I could go to jail if I used a defibrillator that is one day past its expiration date,“ Israel says, “but in Kenya, people don’t have the money for the cheapest model. What nonsense to throw this precious medical equipment away while people in poorer countries die because they desperately need it.“
Israel works at the breakpoint between the laws in a wealthy country like Germany and the reality in the global South. The U.S. and most European countries have strict laws that ban the reuse of medical implants and enforce strict expiration dates for medical equipment. “But if a pacemaker is a few days past its expiration date, it still works perfectly fine,“ Israel says. “And it also happens that we implant a pacemaker and five months later it turns out that the patient needs a different kind. Then we replace it and we’d have to trash the first one in Germany, though it could easily run another 12 years.“
“If we get this right, we have lots of devices we can implant, hips and knees, etcetera. Where this will lead is limitless," says Eva Kline Rogers, the program coordinator for My Heart, Your Heart.
Israel has been collecting donations of pacemakers and defibrillators from manufacturers but also from other doctors and from funeral homes for his nonprofit Pacemakers for East Africa since 2003. Most funeral homes in the U.S. and Europe are legally obliged to remove pacemakers from the dead before cremation. “Most pacemakers survive their owners,“ says Israel. He sterilizes the pacemakers and finds them a new life in East Africa. Studies show that reused pacemakers carry no greater risk for the patients than new ones.
In the U.S., University of Michigan professor Thomas Crawford heads up a similar initiative, My Heart, Your Heart. “Each year 1 to 2 million individuals worldwide die due to a lack of access to pacemakers and defibrillators,” the organization notes on its website. The nonprofit was founded in 2009, but it took four years for the doctors to get permission from the FDA to export pacemakers. Since receiving permission, the organization has sent dozens of devices to the Philippines, Haiti, Venezuela, Kenya, Sierra Leone and Ukraine. “We were the first doctors ever to implant a pacemaker in Sierra Leone in 2018,” says Crawford, who has traveled extensively to most of the recipient countries.
Even individuals can donate their pacemakers; the organization offers a prepaid envelope. “My mother recently passed and she donated her device,” says Tina Alexandris-Souphis, one of the doctors at University of Michigan who collaborates on My Heart, Your Heart. The organization works with World Medical Relief and the U.K. based charity Pace4Life to maintain a registry of the most urgent patients and send devices to where they are needed the most.
My Heart, Your Heart is also conducting a randomized controlled trial to provide further evidence that reused pacemakers pose no additional risk. “Our vision is that we establish this is safe and create a blueprint for organizations around the world to safely reuse these devices instead of them being thrown in the trash,” says Eva Kline Rogers, the program’s coordinator. “If we get this right, we have lots of devices we can implant, hips and knees, etc. Where this will lead is limitless.” She points out that in addition to receiving the donated devices, the doctors in the global South also benefit from the expertise of renowned cardiologists, such as Crawford, who sometimes advise them in complex cases.
And Adrian Baranchuk, a Canadian doctor at the Kingston General Hospital at the Queen’s University, regularly travels through South America with his “cardiology van” to help villagers in remote areas with heart problems.
Israel says that he’s been accused of racism, in thinking that these pacemakers are suitable for those in the global South - many of whom are people of color - even though officials in wealthier countries consider them to be trash. The cardiologist counters such criticism with stories about desperate need of his patients. At his first medical visit to Nairobi that he organized with a local cardiologist, six patients were waiting for him. “In Germany, they would all be considered emergencies,” Israel says. One eighty-year old grandmother had a heartrate of 18. “I’ve never before seen anything like this,” Israel exclaims. “At first I thought I couldn’t find her pulse before I realized that her heart was only beating once every three seconds.” After the surgery, she got up, dressed herself and hurriedly packed her bag, explaining she had a ton of work to accomplish. Her family was in disbelief, Israel says. “They told me she had been bedridden for five years because as soon as she tried to get up she would faint.”

Israel has been accused of racism, in thinking that these pacemakers are suitable for those in the global South even though they're considered to be trash by officials in wealthier countries. The cardiologist counters such criticism with stories about desperate need of his patients.
Carsten Israel
The hospital in Nairobi where Israel conducts the surgeries, charges patients $200 for the use of its facilities. If patients can’t afford that sum, Israel will pay it from the funds of his nonprofit. For some people, $200 far exceeds their resources. Once, a family from the extremely poor Northern region of Kenya told him they couldn’t afford the $3 for the bus ride to Nairobi. Israel suspected this was a pretense because they were afraid of the surgery and agreed to reimburse the $3, “but when they came, they were wearing rags and were so rail-thin, I understood that they really needed every cent they had for food.”
Israel is a renowned cardiologists in Germany. And yet, he considers his work in East Africa to be particularly meaningful. “Generally, most patients in Germany will get the treatment they need,” he says, “and I never before experienced that people have an illness that is easily curable but simply won’t be treated.” He also feels a heavy responsibility. Many patients have his personal cell phone and call him when they have problems or good news about how they’re doing.
Some of those progress reports come much faster than in Israel’s home country. Before he implanted a pacemaker in a tall Massai in Kenya, the man had been picked on by his family because he wouldn’t help much with the hard work on the family peanut farm. “When I examined him, he had a pulse of 40,” Israel says. “It’s a miracle he was even standing upright, let alone hauling heavy bags.” After the surgery, Israel advised his patient to stay the night for observation, but the patient couldn’t wait to leave. Two hours later, he returned, covered in sweat. He’d been running sprints with his brothers to test the new device. Israel shakes his head. In Germany, it would be unthinkable for a patient to engage in athletics immediately after surgery. But the patient was exuberant: “I was the fastest!”
The success stories are notable partly because the challenges remain so steep. In Zambia, for instance, there is a single cardiologist; she determined to become one after losing her younger sister to an easily curable heart disease. Often, the hospitals not only lack pacemakers but also sterile surgery equipment, antibiotics and other essential material. Therefore, Israel and his team import everything they need for the surgeries, including medication. If necessary, they improvise. “I’ve done surgery with a desk lamp hanging from the ceiling by threads,” Israel says. He already knows that he will need to return to Kenya in six months to replace the pacemaker of one of his patients and replace the batteries in others. If he doesn’t travel, lives are at risk.