Are the gains from gain-of-function research worth the risks?
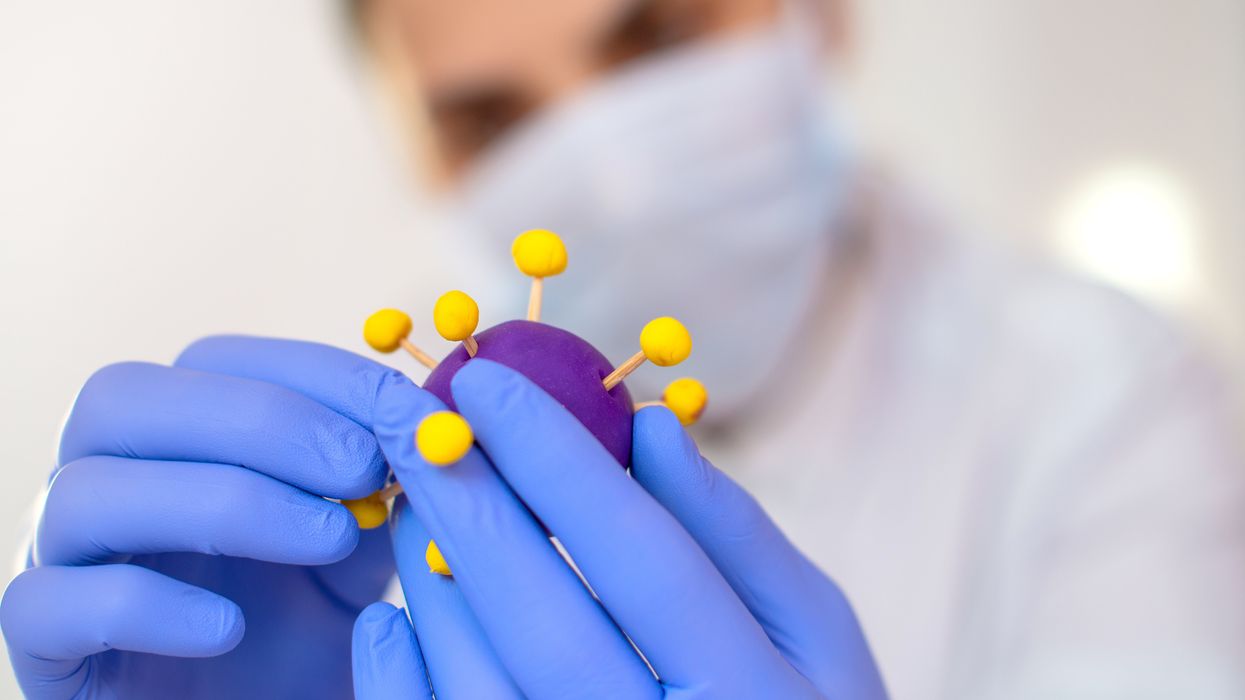
Gain-of-function research can make pathogens more infectious and deadly. It also enables scientists to prepare remedies in advance.
Scientists have long argued that gain-of-function research, which can make viruses and other infectious agents more contagious or more deadly, was necessary to develop therapies and vaccines to counter the pathogens in case they were used for biological warfare. As the SARS-CoV-2 origins are being investigated, one prominent theory suggests it had leaked from a biolab that conducted gain-of-function research, causing a global pandemic that claimed nearly 6.9 million lives. Now some question the wisdom of engaging in this type of research, stating that the risks may far outweigh the benefits.
“Gain-of-function research means genetically changing a genome in a way that might enhance the biological function of its genes, such as its transmissibility or the range of hosts it can infect,” says George Church, professor of genetics at Harvard Medical School. This can occur through direct genetic manipulation as well as by encouraging mutations while growing successive generations of micro-organism in culture. “Some of these changes may impact pathogenesis in a way that is hard to anticipate in advance,” Church says.
In the wake of the global pandemic, the pros and cons of gain-of-function research are being fiercely debated. Some scientists say this type of research is vital for preventing future pandemics or for preparing for bioweapon attacks. Others consider it another disaster waiting to happen. The Government Accounting Office issued a report charging that a framework developed by the U.S. Department of Health & Human Services (HHS) provided inadequate oversight of this potentially deadly research. There’s a movement to stop it altogether. In January, the Viral Gain-of-Function Research Moratorium Act (S. 81) was introduced into the Senate to cease awarding federal research funding to institutions doing gain-of-function studies.
While testifying before the House COVID Origins Select Committee on March 8th, Robert Redfield, former director of the U.S. Centers for Disease Control and Prevention, said that COVID-19 may have resulted from an accidental lab leak involving gain-of-function research. Redfield said his conclusion is based upon the “rapid and high infectivity for human-to-human transmission, which then predicts the rapid evolution of new variants.”
“It is a very, very, very small subset of life science research that could potentially generate a potential pandemic pathogen,” said Gerald Parker, associate dean for Global One Health at Texas A&M University.
“In my opinion,” Redfield continues, “the COVID-19 pandemic presents a case study on the potential dangers of such research. While many believe that gain-of-function research is critical to get ahead of viruses by developing vaccines, in this case, I believe that was the exact opposite.” Consequently, Redfield called for a moratorium on gain-of-function research until there is consensus about the value of such risky science.
What constitutes risky?
The Federal Select Agent Program lists 68 specific infectious agents as risky because they are either very contagious or very deadly. In order to work with these 68 agents, scientists must register with the federal government. Meanwhile, research on deadly pathogens that aren’t easily transmitted, or pathogens that are quite contagious but not deadly, can be conducted without such oversight. “If you’re not working with select agents, you’re not required to register the research with the federal government,” says Gerald Parker, associate dean for Global One Health at Texas A&M University. But the 68-item list may not have everything that could possibly become dangerous or be engineered to be dangerous, thus escaping the government’s scrutiny—an issue that new regulations aim to address.
In January 2017, the White House Office of Science and Technology Policy (OSTP) issued additional guidance. It required federal departments and agencies to follow a series of steps when reviewing proposed research that could create, transfer, or use potential pandemic pathogens resulting from the enhancement of a pathogen’s transmissibility or virulence in humans.
In defining risky pathogens, OSTP included viruses that were likely to be highly transmissible and highly virulent, and thus very deadly. The Proposed Biosecurity Oversight Framework for the Future of Science, outlined in 2023, broadened the scope to require federal review of research “that is reasonably anticipated to enhance the transmissibility and/or virulence of any pathogen” likely to pose a threat to public health, health systems or national security. Those types of experiments also include the pathogens’ ability to evade vaccines or therapeutics, or diagnostic detection.
However, Parker says that dangers of generating a pandemic-level germ are tiny. “It is a very, very, very small subset of life science research that could potentially generate a potential pandemic pathogen.” Since gain-of-function guidelines were first issued in 2017, only three such research projects have met those requirements for HHS review. They aimed to study influenza and bird flu. Only two of those projects were funded, according to the NIH Office of Science Policy. For context, NIH funded approximately 11,000 of the 54,000 grant applications it received in 2022.
Guidelines governing gain-of-function research are being strengthened, but Church points out they aren’t ideal yet. “They need to be much clearer about penalties and avoiding positive uses before they would be enforceable.”
What do we gain from gain-of-function research?
The most commonly cited reason to conduct gain-of-function research is for biodefense—the government’s ability to deal with organisms that may pose threats to public health.
In the era of mRNA vaccines, the advance preparedness argument may be even less relevant.
“The need to work with potentially dangerous viruses is central to our preparedness,” Parker says. “It’s essential that we know and understand the basic biology, microbiology, etc. of some of these dangerous pathogens.” That includes increasing our knowledge of the molecular mechanisms by which a virus could become a sustained threat to humans. “Knowing that could help us detect [risks] earlier,” Parker says—and could make it possible to have medical countermeasures, like vaccines and therapeutics, ready.
Most vaccines, however, aren’t affected by this type of research. Essentially, scientists hope they will never need to use it. Moreover, Paul Mango, HSS former deputy chief of staff for policy, and author of the 2022 book Warp Speed, says he believes that in the era of mRNA vaccines, the advance preparedness argument may be even less relevant. “That’s because these vaccines can be developed and produced in less than 12 months, unlike traditional vaccines that require years of development,” he says.
Can better oversight guarantee safety?
Another situation, which Parker calls unnecessarily dangerous, is when regulatory bodies cannot verify that the appropriate biosafety and biosecurity controls are in place.
Gain-of-function studies, Parker points out, are conducted at the basic research level, and they’re performed in high-containment labs. “As long as all the processes, procedures and protocols are followed and there’s appropriate oversight at the institutional and scientific level, it can be conducted safely.”
Globally, there are 69 Biosafety Level 4 (BSL4) labs operating, under construction or being planned, according to recent research from King’s College London and George Mason University for Global BioLabs. Eleven of these 18 high-containment facilities that are planned or under construction are in Asia. Overall, three-quarters of the BSL4 labs are in cities, increasing public health risks if leaks occur.
Researchers say they are confident in the oversight system for BSL4 labs within the U.S. They are less confident in international labs. Global BioLabs’ report concurs. It gives the highest scores for biosafety to industrialized nations, led by France, Australia, Canada, the U.S. and Japan, and the lowest scores to Saudi Arabia, India and some developing African nations. Scores for biosecurity followed similar patterns.
“There are no harmonized international biosafety and biosecurity standards,” Parker notes. That issue has been discussed for at least a decade. Now, in the wake of SARS and the COVID-19 pandemic, scientists and regulators are likely to push for unified oversight standards. “It’s time we got serious about international harmonization of biosafety and biosecurity standards and guidelines,” Parker says. New guidelines are being worked on. The National Science Advisory Board for Biosecurity (NSABB) outlined its proposed recommendations in the document titled Proposed Biosecurity Oversight Framework for the Future of Science.
The debates about whether gain-of-function research is useful or poses unnecessary risks to humanity are likely to rage on for a while. The public too has a voice in this debate and should weigh in by communicating with their representatives in government, or by partaking in educational forums or initiatives offered by universities and other institutions. In the meantime, scientists should focus on improving the research regulations, Parker notes. “We need to continue to look for lessons learned and for gaps in our oversight system,” he says. “That’s what we need to do right now.”
On May 13th, scientific and medical experts will discuss and answer questions about the vaccine for those under 16.
This virtual event convened leading scientific and medical experts to address the public's questions and concerns about Covid-19 vaccines in kids and teens. Highlight video below.
DATE:
Thursday, May 13th, 2021
12:30 p.m. - 1:45 p.m. EDT
Dr. H. Dele Davies, M.D., MHCM

Senior Vice Chancellor for Academic Affairs and Dean for Graduate Studies at the University of Nebraska Medical (UNMC). He is an internationally recognized expert in pediatric infectious diseases and a leader in community health.
Dr. Emily Oster, Ph.D.

Professor of Economics at Brown University. She is a best-selling author and parenting guru who has pioneered a method of assessing school safety.
Dr. Tina Q. Tan, M.D.

Professor of Pediatrics at the Feinberg School of Medicine, Northwestern University. She has been involved in several vaccine survey studies that examine the awareness, acceptance, barriers and utilization of recommended preventative vaccines.
Dr. Inci Yildirim, M.D., Ph.D., M.Sc.

Associate Professor of Pediatrics (Infectious Disease); Medical Director, Transplant Infectious Diseases at Yale School of Medicine; Associate Professor of Global Health, Yale Institute for Global Health. She is an investigator for the multi-institutional COVID-19 Prevention Network's (CoVPN) Moderna mRNA-1273 clinical trial for children 6 months to 12 years of age.
About the Event Series
This event is the second of a four-part series co-hosted by Leaps.org, the Aspen Institute Science & Society Program, and the Sabin–Aspen Vaccine Science & Policy Group, with generous support from the Gordon and Betty Moore Foundation and the Howard Hughes Medical Institute.
:
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
Pregnant & Breastfeeding Women Who Get the COVID-19 Vaccine Are Protecting Their Infants, Research Suggests
Becky Cummings, who got vaccinated in December, snuggles her newborn, Clark, while he takes a nap.
Becky Cummings had multiple reasons to get vaccinated against COVID-19 while tending to her firstborn, Clark, who arrived in September 2020 at 27 weeks.
The 29-year-old intensive care unit nurse in Greensboro, North Carolina, had witnessed the devastation day in and day out as the virus took its toll on the young and old. But when she was offered the vaccine, she hesitated, skeptical of its rapid emergency use authorization.
Exclusion of pregnant and lactating mothers from clinical trials fueled her concerns. Ultimately, though, she concluded the benefits of vaccination outweighed the risks of contracting the potentially deadly virus.
"Long story short," Cummings says, in December "I got vaccinated to protect myself, my family, my patients, and the general public."
At the time, Cummings remained on the fence about breastfeeding, citing a lack of evidence to support its safety after vaccination, so she pumped and stashed breast milk in the freezer. Her son is adjusting to life as a preemie, requiring mother's milk to be thickened with formula, but she's becoming comfortable with the idea of breastfeeding as more research suggests it's safe.
"If I could pop him on the boob," she says, "I would do it in a heartbeat."
Now, a study recently published in the Journal of the American Medical Association found "robust secretion" of specific antibodies in the breast milk of mothers who received a COVID-19 vaccine, indicating a potentially protective effect against infection in their infants.
The presence of antibodies in the breast milk, detectable as early as two weeks after vaccination, lasted for six weeks after the second dose of the Pfizer-BioNTech vaccine.
"We believe antibody secretion into breast milk will persist for much longer than six weeks, but we first wanted to prove any secretion at all after vaccination," says Ilan Youngster, the study's corresponding author and head of pediatric infectious diseases at Shamir Medical Center in Zerifin, Israel.
That's why the research team performed a preliminary analysis at six weeks. "We are still collecting samples from participants and hope to soon be able to comment about the duration of secretion."
As with other respiratory illnesses, such as influenza and pertussis, secretion of antibodies in breast milk confers protection from infection in infants. The researchers expect a similar immune response from the COVID-19 vaccine and are expecting the findings to spur an increase in vaccine acceptance among pregnant and lactating women.
A COVID-19 outbreak struck three families the research team followed in the study, resulting in at least one non-breastfed sibling developing symptomatic infection; however, none of the breastfed babies became ill. "This is obviously not empirical proof," Youngster acknowledges, "but still a nice anecdote."
Leaps.org inquired whether infants who derive antibodies only through breast milk are likely to have a lower immunity than infants whose mothers were vaccinated while they were in utero. In other words, is maternal transmission of antibodies stronger during pregnancy than during breastfeeding, or about the same?
"This is a different kind of transmission," Youngster explains. "When a woman is infected or vaccinated during pregnancy, some antibodies will be transferred through the placenta to the baby's bloodstream and be present for several months." But in the nursing mother, that protection occurs through local action. "We always recommend breastfeeding whenever possible, and, in this case, it might have added benefits."
A study published online in March found COVID-19 vaccination provided pregnant and lactating women with robust immune responses comparable to those experienced by their nonpregnant counterparts. The study, appearing in the American Journal of Obstetrics and Gynecology, documented the presence of vaccine-generated antibodies in umbilical cord blood and breast milk after mothers had been vaccinated.
Natali Aziz, a maternal-fetal medicine specialist at Stanford University School of Medicine, notes that it's too early to draw firm conclusions about the reduction in COVID-19 infection rates among newborns of vaccinated mothers. Citing the two aforementioned research studies, she says it's biologically plausible that antibodies passed through the placenta and breast milk impart protective benefits. While thousands of pregnant and lactating women have been vaccinated against COVID-19, without incurring adverse outcomes, many are still wondering whether it's safe to breastfeed afterward.
It's important to bear in mind that pregnant women may develop more severe COVID-19 complications, which could lead to intubation or admittance to the intensive care unit. "We, in our practice, are supporting pregnant and breastfeeding patients to be vaccinated," says Aziz, who is also director of perinatal infectious diseases at Stanford Children's Health, which has been vaccinating new mothers and other hospitalized patients at discharge since late April.
Earlier in April, Huntington Hospital in Long Island, New York, began offering the COVID-19 vaccine to women after they gave birth. The hospital chose the one-shot Johnson & Johnson vaccine for postpartum patients, so they wouldn't need to return for a second shot while acclimating to life with a newborn, says Mitchell Kramer, chairman of obstetrics and gynecology.
The hospital suspended the program when the Food and Drug Administration and the Centers for Disease Control and Prevention paused use of the J&J vaccine starting April 13, while investigating several reports of dangerous blood clots and low platelet counts among more than 7 million people in the United States who had received that vaccine.
In lifting the pause April 23, the agencies announced the vaccine's fact sheets will bear a warning of the heightened risk for a rare but serious blood clot disorder among women under age 50. As a result, Kramer says, "we will likely not be using the J&J vaccine for our postpartum population."
So, would it make sense to vaccinate infants when one for them eventually becomes available, not just their mothers? "In general, most of the time, infants do not have as good of an immune response to vaccines," says Jonathan Temte, associate dean for public health and community engagement at the University of Wisconsin School of Medicine and Public Health in Madison.
"Many of our vaccines are held until children are six months of age. For example, the influenza vaccine starts at age six months, the measles vaccine typically starts one year of age, as do rubella and mumps. Immune response is typically not very good for viral illnesses in young infants under the age of six months."
So far, the FDA has granted emergency use authorization of the Pfizer-BioNTech vaccine for children as young as 16 years old. The agency is considering data from Pfizer to lower that age limit to 12. Studies are also underway in children under age 12. Meanwhile, data from Moderna on 12-to 17-year-olds and from Pfizer on 12- to 15-year-olds have not been made public. (Pfizer announced at the end of March that its vaccine is 100 percent effective in preventing COVID-19 in the latter age group, and FDA authorization for this population is expected soon.)
"There will be step-wise progression to younger children, with infants and toddlers being the last ones tested," says James Campbell, a pediatric infectious diseases physician and head of maternal and child clinical studies at the University of Maryland School of Medicine Center for Vaccine Development.
"Once the data are analyzed for safety, tolerability, optimal dose and regimen, and immune responses," he adds, "they could be authorized and recommended and made available to American children." The data on younger children are not expected until the end of this year, with regulatory authorization possible in early 2022.
For now, Vonnie Cesar, a family nurse practitioner in Smyrna, Georgia, is aiming to persuade expectant and new mothers to get vaccinated. She has observed that patients in metro Atlanta seem more inclined than their rural counterparts.
To quell some of their skepticism and fears, Cesar, who also teaches nursing students, conceived a visual way to demonstrate the novel mechanism behind the COVID-19 vaccine technology. Holding a palm-size physical therapy ball outfitted with clear-colored push pins, she simulates the spiked protein of the coronavirus. Slime slathered at the gaps permeates areas around the spikes—a process similar to how our antibodies build immunity to the virus.
These conversations often lead hesitant patients to discuss vaccination with their husbands or partners. "The majority of people I'm speaking with," she says, "are coming to the conclusion that this is the right thing for me, this is the common good, and they want to make sure that they're here for their children."
CORRECTION: An earlier version of this article mistakenly stated that the COVID-19 vaccines were granted emergency "approval." They have been granted emergency use authorization, not full FDA approval. We regret the error.

