The Scientist Behind the Pap Smear Saved Countless Women from Cervical Cancer

George Papanicolaou (1883-1962), Greek-born American physician developed a simple cytological test for cervical cancer in 1928.
For decades, women around the world have made the annual pilgrimage to their doctor for the dreaded but potentially life-saving Papanicolaou test, a gynecological exam to screen for cervical cancer named for Georgios Papanicolaou, the Greek immigrant who developed it.
The Pap smear, as it is commonly known, is credited for reducing cervical cancer mortality by 70% since the 1960s; the American Cancer Society (ACS) still ranks the Pap as the most successful screening test for preventing serious malignancies. Nonetheless, the agency, as well as other medical panels, including the US Preventive Services Task Force and the American College of Obstetrics and Gynecology are making a strong push to replace the Pap with the more sensitive high-risk HPV screening test for the human papillomavirus virus, which causes nearly all cases of cervical cancer.
So, how was the Pap developed and how did it become the gold standard of cervical cancer detection for more than 60 years?
Born on May 13, 1883, on the island of Euboea, Greece, Georgios Papanicolaou attended the University of Athens where he majored in music and the humanities before earning his medical degree in 1904 and PhD from the University of Munich six years later. In Europe, Papanicolaou was an assistant military surgeon during the Balkan War, a psychologist for an expedition of the Oceanographic Institute of Monaco and a caregiver for leprosy patients.
When he and his wife, Andromache Mavroyenous (Mary), arrived at Ellis Island on October 19, 1913, the young couple had scarcely more than the $250 minimum required to immigrate, spoke no English and had no job prospects. They worked a series of menial jobs--department store sales clerk, rug salesman, newspaper clerk, restaurant violinist--before Papanicolaou landed a position as an anatomy assistant at Cornell University and Mary was hired as his lab assistant, an arrangement that would last for the next 50 years.
Papanikolaou would later say the discovery "was one of the greatest thrills I ever experienced during my scientific career."
In his early research, Papanikolaou used guinea pigs to prove that gender is determined by the X and Y chromosomes. Using a pediatric nasal speculum, he collected and microscopically examined vaginal secretions of guinea pigs, which revealed distinct cell changes connected to the menstrual cycle. He moved on to study reproductive patterns in humans, beginning with his faithful wife, Mary, who not only endured his almost-daily cervical exams for decades, but also recruited friends as early research participants.
Writing in the medical journal Growth in 1920, the scientist outlined his theory that a microscopic smear of vaginal fluid could detect the presence of cancer cells in the uterus. Papanikolaou would later say the discovery "was one of the greatest thrills I ever experienced during my scientific career."
At this time, cervical cancer was the number one cancer killer of American women but physicians were skeptical of these new findings. They continued to rely on biopsy and curettage to diagnose and treat the disease until Papanicolaou's discovery was published in American Journal of Obstetrics and Gynecology. An inexpensive, easy-to-perform test that could detect cervical cancer, precancerous dysplasia and other cytological diseases was a sea change. Between 1975 and 2001, the cervical cancer rate was cut in half.
Papanicolaou became Emeritus Professor at Cornell University Medical College and received numerous awards, including the Albert Lasker Award for Clinical Medical Research and the Medal of Honor from the American Cancer Society. His image was featured on the Greek currency and the US Post Office issued a commemorative stamp in his honor. But international acclaim didn't lead to a more relaxed schedule. The researcher continued to work seven days a week and refused to take vacations.
After nearly 50 years, Papanicolaou left Cornell to head and develop the Cancer Institute of Miami. He died of a heart attack on February 19, 1962, just three months after his arrival. Mary continued to work in the renamed Papanicolaou Cancer Research Institute until her death 20 years later.
The annual pap smear was originally tied to renewing a birth control prescription. Canada began recommending Pap exams every three years in 1978. The United States followed suit in 2012, noting that it takes many years for cervical cancer to develop. In September 2020, the American Cancer Society recommended delaying the first gynecological pelvic exam until age 25 and replacing the Pap test completely with the more accurate human papillomavirus (HPV) test every five years as the technology becomes more widely available.
Not everyone agrees that it's time to do away with this proven screening method, though. The incidence rate of cervical cancer among Hispanic women is 28% higher than for white women, and Black women are more likely to die of cervical cancer than any other racial or ethnicities.
Whether the Pap is administered every year, every three years or not at all, Papanicolaou will always be known as the medical hero who saved countless women who would otherwise have succumbed to cervical cancer.
Two students had an idea at a scrapyard. They went on to "upcycle" 80,000 airbags, 100,000 seatbelts and 28,000 belt buckles – the equivalent of 60 tons of car trash
Luckily, two college freshmen at the Rotterdam School of Management, Erasmus University, were naïve enough to take their bicycles to the scrapyard. In a previous stroke of fortune, the freshmen, Adrian Goosses and Michael Widmann, had been assigned as roommates and had quickly hit it off. Now they were looking for a cool recycling project for their first semester “strategic entrepreneurship” course—maybe they could turn old tires into comfortable lounge chairs, they thought.
“Everybody gets around by bike in Rotterdam,” says Goosses, now 32, from his home in Cologne, Germany. “The tires were way too heavy and cumbersome to transport by bike,” Widmann chimes in via Zoom from Bolzano, Italy, where he lives.
Sifting through the car trash for something handier led the two students to an idea that has since flourished: Could the airbag and seatbelts from a banged up compact car be salvaged and turned into a sustainable backpack? The size of the airbag was already a natural fit. The seatbelts made perfect shoulder straps. After returning from the scrapyard, “We stitched the prototype together by hand with a needle and yarn,” says Goosses. “Yet we didn’t even know how to sew!”
Much to their surprise, their classmates responded with so much enthusiasm to their “trash bag” concept that it convinced the two innovators to keep going. Every semester, they improved the prototype further. With the help of YouTube videos, they taught themselves how to sew. Because modern electric sewing machines had a difficult time breaking through the tough nylon of the airbags, Goosses and Widmann went to a second-hand shop and purchased an ancient Singer from 1880 for 10 Euros. They dyed the first airbags in a saucepan in the garden outside of the apartment they shared.
“By the time we graduated, we had a presentable prototype and a business plan,” Goosses says.
Despite their progress, Goosses and Widmann are up against a problem that’s immense: Cars are notoriously difficult to recycle because many parts are considered toxic waste.
It’s an example of “upcycling,” when you spot a potential new use in something that’s been trashed, shelved or otherwise retired. The approach has received increasing attention and support from the U.S. Environmental Protection Agency and others to boost sustainability in all kinds of areas, from fashion (where even luxury brands like Balenciaga or Coach repurpose vintage clothing and bags) to architecture, where reusing wood, steel and bricks significantly reduces a building’s carbon footprint.
In addition to helping the planet, those who do it well can make a living from it. These days, Goosses and Widmann own a flourishing company: Airpaq. A crowdfunding campaign in 2017 yielded 70,000 Euros to get them started. Since then, they have upcycled 80,000 airbags, 100,000 seatbelts and 28,000 belt buckles – the equivalent of 60 tons of car trash.
For the successful upcycling, they received the 2021 German Design Award and, earlier this year, the renowned German Sustainability Award. The jurors evaluating the product commented that the startup “convinced us not only because of their uncompromising quality and functionality but also because of their ecological and ethical values….How well the startup translates upcycling and green fashion into an urban lifestyle brand is impressive.”
Despite their progress, Goosses and Widmann are up against a problem that’s immense: Cars are notoriously difficult to recycle because many parts are considered toxic waste. Therefore, up to 25% of vehicle scraps get shredded every year in Germany alone, the equivalent of over 501,000 tons. Because airbags and seatbelts are nearly indestructible, they are costly to recycle and almost always end up in landfills. Given that airbags and seatbelts save lives, they are subject to stringent security regulations, and manufacturers have a sky-high reject rate. “If a tiny filament protrudes somewhere, the manufacturer will throw out the entire output,” Widmann explains.
The nearly indestructible qualities that make this material very difficult to recycle render it an excellent resource for backpacks. “The material is so durable, you almost cannot tear it,” Goosses adds and demonstrates with a hard tug that even when the material already has a hole, it won’t rip it further. The material is also water repellent and extremely light.
The antique Singer is still in their Cologne headquarters but only as decoration. Their company with 12 employees is producing 500 backpacks and fanny packs every week in Romania, where the parts are professionally cut by laser, dyed and sewed. Airpaq still procures the belt buckles at scrapyards but they get most of the airbags directly from the reject pile of a nearby airbag producer. “We process the materials where they are produced,” Goosses explains. Only about 15 miles lie between one of Europe’s biggest airbag manufacturers and the Airpaq seamsters in Romania.

Co-founders Adrian Goosses and Michael Widmann demonstrate their company's equation: airbag plus seatbelt equals a backpack that's durable and eco-friendly.
Airpaq
The founders are aware that with price tags ranging from 100 to 160 Euro - a cost that reflects their intensive production process - Airpaq’s bags are hardly competitive. After all, anybody can buy a discount backpack for a fraction of the cost. So they recently added fanny packs for 30 Euro to their product line. Goosses and Widmann know they will need to lower their prices in the long run if they want to expand. Among other things, they didn’t pay themselves salaries during the first two years after founding the company.
Money-making isn’t their only objective. “Of course, it would be cheaper if we did what almost all textile producers do and move production to Asia,” Goosses says. That wasn’t an option for him. “Ship trash to Vietnam and let seamsters sew it together for cheap? No way, that would be anything but sustainable,” he says.
Michael Widmann’s family was already operating a textile production in Romania, mainly producing thin, elastic sports fashion. The family allowed Widmann and Goosses to produce their first professional prototypes there, but then the two youngsters had to buy their own machines, acquire the necessary knowhow, and hire their staff. They both moved to Romania for six months “to get to know the people behind the machines.” The founders emphasize that they pay fair wages, use eco-certified dyes and clean their own wastewater. “Normal production uses five to six liters of water per kilo material,” Widmann explains. “We only need a fraction because we massage the dye into the material by hand: 100 ml water for washing and dying per kilo.”
However, every time they return to the scrapyard, the abundance of trash sparks new ideas. “When you see how much material ends up there…” Widmann says, shaking his head without finishing the sentence. Goosses picks up the train of thought: “We want to make upcycling the new standard. You just have to be creative to get upcycling into the mainstream.”
And maybe they’ll return to their roots and finally find an idea for the tires after all. “One could turn the rubber into soles for comfortable shoes,” Widmann thinks out loud.
Recent advancements in engineering mean that the first preclinical trials for an artificial kidney could happen as soon as 18 months from now
Like all those whose kidneys have failed, Scott Burton’s life revolves around dialysis. For nearly two decades, Burton has been hooked up (or, since 2020, has hooked himself up at home) to a dialysis machine that performs the job his kidneys normally would. The process is arduous, time-consuming, and expensive. Except for a brief window before his body rejected a kidney transplant, Burton has depended on machines to take the place of his kidneys since he was 12-years-old. His whole life, the 39-year-old says, revolves around dialysis.
“Whenever I try to plan anything, I also have to plan my dialysis,” says Burton says, who works as a freelance videographer and editor. “It’s a full-time job in itself.”
Many of those on dialysis are in line for a kidney transplant that would allow them to trade thrice-weekly dialysis and strict dietary limits for a lifetime of immunosuppressants. Burton’s previous transplant means that his body will likely reject another donated kidney unless it matches perfectly—something he’s not counting on. It’s why he’s enthusiastic about the development of artificial kidneys, small wearable or implantable devices that would do the job of a healthy kidney while giving users like Burton more flexibility for traveling, working, and more.
Still, the devices aren’t ready for testing in humans—yet. But recent advancements in engineering mean that the first preclinical trials for an artificial kidney could happen as soon as 18 months from now, according to Jonathan Himmelfarb, a nephrologist at the University of Washington.
“It would liberate people with kidney failure,” Himmelfarb says.
An engineering marvel
Compared to the heart or the brain, the kidney doesn’t get as much respect from the medical profession, but its job is far more complex. “It does hundreds of different things,” says UCLA’s Ira Kurtz.
Kurtz would know. He’s worked as a nephrologist for 37 years, devoting his career to helping those with kidney disease. While his colleagues in cardiology and endocrinology have seen major advances in the development of artificial hearts and insulin pumps, little has changed for patients on hemodialysis. The machines remain bulky and require large volumes of a liquid called dialysate to remove toxins from a patient’s blood, along with gallons of purified water. A kidney transplant is the next best thing to someone’s own, functioning organ, but with over 600,000 Americans on dialysis and only about 100,000 kidney transplants each year, most of those in kidney failure are stuck on dialysis.
Part of the lack of progress in artificial kidney design is the sheer complexity of the kidney’s job. Each of the 45 different cell types in the kidney do something different.
Part of the lack of progress in artificial kidney design is the sheer complexity of the kidney’s job. To build an artificial heart, Kurtz says, you basically need to engineer a pump. An artificial pancreas needs to balance blood sugar levels with insulin secretion. While neither of these tasks is simple, they are fairly straightforward. The kidney, on the other hand, does more than get rid of waste products like urea and other toxins. Each of the 45 different cell types in the kidney do something different, helping to regulate electrolytes like sodium, potassium, and phosphorous; maintaining blood pressure and water balance; guiding the body’s hormonal and inflammatory responses; and aiding in the formation of red blood cells.

There's been little progress for patients during Ira Kurtz's 37 years as a nephrologist. Artificial kidneys would change that.
UCLA
Dialysis primarily filters waste, and does so well enough to keep someone alive, but it isn’t a true artificial kidney because it doesn’t perform the kidney’s other jobs, according to Kurtz, such as sensing levels of toxins, wastes, and electrolytes in the blood. Due to the size and water requirements of existing dialysis machines, the equipment isn’t portable. Physicians write a prescription for a certain duration of dialysis and assess how well it’s working with semi-regular blood tests. The process of dialysis itself, however, is conducted blind. Doctors can’t tell how much dialysis a patient needs based on kidney values at the time of treatment, says Meera Harhay, a nephrologist at Drexel University in Philadelphia.
But it’s the impact of dialysis on their day-to-day lives that creates the most problems for patients. Only one-quarter of those on dialysis are able to remain employed (compared to 85% of similar-aged adults), and many report a low quality of life. Having more flexibility in life would make a major different to her patients, Harhay says.
“Almost half their week is taken up by the burden of their treatment. It really eats away at their freedom and their ability to do things that add value to their life,” she says.
Art imitates life
The challenge for artificial kidney designers was how to compress the kidney’s natural functions into a portable, wearable, or implantable device that wouldn’t need constant access to gallons of purified and sterilized water. The other universal challenge they faced was ensuring that any part of the artificial kidney that would come in contact with blood was kept germ-free to prevent infection.
As part of last year’s KidneyX Prize, a partnership between the U.S. Department of Health and Human Services and the American Society of Nephrology, inventors were challenged to create prototypes for artificial kidneys. Himmelfarb’s team at the University of Washington’s Center for Dialysis Innovation won the prize by focusing on miniaturizing existing technologies to create a portable dialysis machine. The backpack sized AKTIV device (Ambulatory Kidney to Increase Vitality) will recycle dialysate in a closed loop system that removes urea from blood and uses light-based chemical reactions to convert the urea to nitrogen and carbon dioxide, which allows the dialysate to be recirculated.
Himmelfarb says that the AKTIV can be used when at home, work, or traveling, which will give users more flexibility and freedom. “If you had a 30-pound device that you could put in the overhead bins when traveling, you could go visit your grandkids,” he says.
Kurtz’s team at UCLA partnered with the U.S. Kidney Research Corporation and Arkansas University to develop a dialysate-free desktop device (about the size of a small printer) as the first phase of a progression that will he hopes will lead to something small and implantable. Part of the reason for the artificial kidney’s size, Kurtz says, is the number of functions his team are cramming into it. Not only will it filter urea from blood, but it will also use electricity to help regulate electrolyte levels in a process called electrodeionization. Kurtz emphasizes that these additional functions are what makes his design a true artificial kidney instead of just a small dialysis machine.
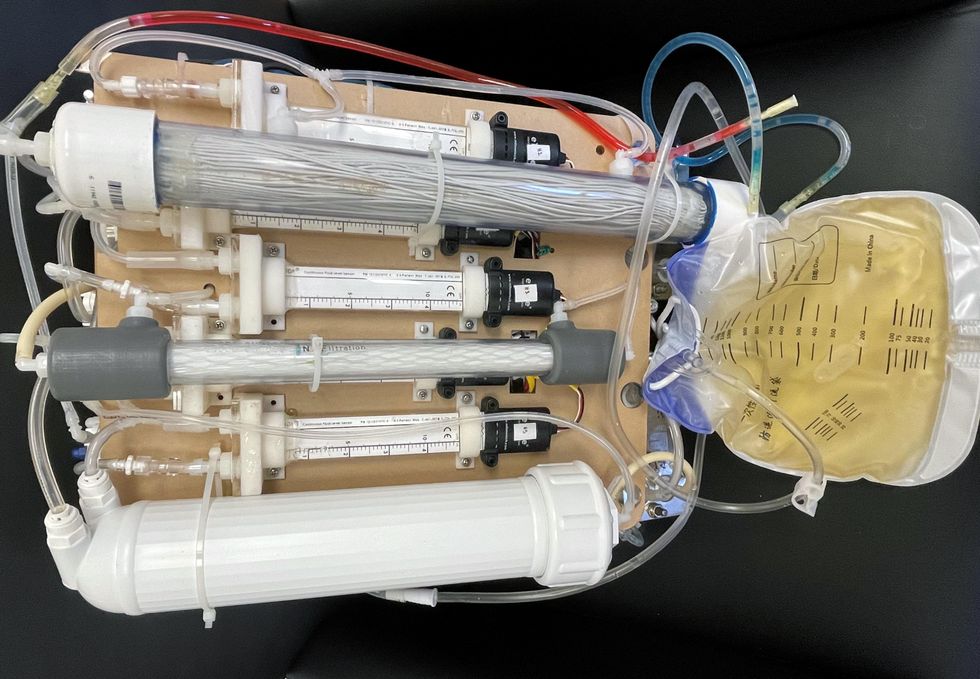
One version of an artificial kidney.
UCLA
“It doesn't have just a static function. It has a bank of sensors that measure chemicals in the blood and feeds that information back to the device,” Kurtz says.
Other startups are getting in on the game. Nephria Bio, a spinout from the South Korean-based EOFlow, is working to develop a wearable dialysis device, akin to an insulin pump, that uses miniature cartridges with nanomaterial filters to clean blood (Harhay is a scientific advisor to Nephria). Ian Welsford, Nephria’s co-founder and CTO, says that the device’s design means that it can also be used to treat acute kidney injuries in resource-limited settings. These potentials have garnered interest and investment in artificial kidneys from the U.S. Department of Defense.
For his part, Burton is most interested in an implantable device, as that would give him the most freedom. Even having a regular outpatient procedure to change batteries or filters would be a minor inconvenience to him.
“Being plugged into a machine, that’s not mimicking life,” he says.