How a Deadly Fire Gave Birth to Modern Medicine

The Cocoanut Grove fire in Boston in 1942 tragically claimed 490 lives, but was the catalyst for several important medical advances.
On the evening of November 28, 1942, more than 1,000 revelers from the Boston College-Holy Cross football game jammed into the Cocoanut Grove, Boston's oldest nightclub. When a spark from faulty wiring accidently ignited an artificial palm tree, the packed nightspot, which was only designed to accommodate about 500 people, was quickly engulfed in flames. In the ensuing panic, hundreds of people were trapped inside, with most exit doors locked. Bodies piled up by the only open entrance, jamming the exits, and 490 people ultimately died in the worst fire in the country in forty years.
"People couldn't get out," says Dr. Kenneth Marshall, a retired plastic surgeon in Boston and president of the Cocoanut Grove Memorial Committee. "It was a tragedy of mammoth proportions."
Within a half an hour of the start of the blaze, the Red Cross mobilized more than five hundred volunteers in what one newspaper called a "Rehearsal for Possible Blitz." The mayor of Boston imposed martial law. More than 300 victims—many of whom subsequently died--were taken to Boston City Hospital in one hour, averaging one victim every eleven seconds, while Massachusetts General Hospital admitted 114 victims in two hours. In the hospitals, 220 victims clung precariously to life, in agonizing pain from massive burns, their bodies ravaged by infection.

The scene of the fire.
Boston Public Library
Tragic Losses Prompted Revolutionary Leaps
But there is a silver lining: this horrific disaster prompted dramatic changes in safety regulations to prevent another catastrophe of this magnitude and led to the development of medical techniques that eventually saved millions of lives. It transformed burn care treatment and the use of plasma on burn victims, but most importantly, it introduced to the public a new wonder drug that revolutionized medicine, midwifed the birth of the modern pharmaceutical industry, and nearly doubled life expectancy, from 48 years at the turn of the 20th century to 78 years in the post-World War II years.
The devastating grief of the survivors also led to the first published study of post-traumatic stress disorder by pioneering psychiatrist Alexandra Adler, daughter of famed Viennese psychoanalyst Alfred Adler, who was a student of Freud. Dr. Adler studied the anxiety and depression that followed this catastrophe, according to the New York Times, and "later applied her findings to the treatment World War II veterans."
Dr. Ken Marshall is intimately familiar with the lingering psychological trauma of enduring such a disaster. His mother, an Irish immigrant and a nurse in the surgical wards at Boston City Hospital, was on duty that cold Thanksgiving weekend night, and didn't come home for four days. "For years afterward, she'd wake up screaming in the middle of the night," recalls Dr. Marshall, who was four years old at the time. "Seeing all those bodies lined up in neat rows across the City Hospital's parking lot, still in their evening clothes. It was always on her mind and memories of the horrors plagued her for the rest of her life."
The sheer magnitude of casualties prompted overwhelmed physicians to try experimental new procedures that were later successfully used to treat thousands of battlefield casualties. Instead of cutting off blisters and using dyes and tannic acid to treat burned tissues, which can harden the skin, they applied gauze coated with petroleum jelly. Doctors also refined the formula for using plasma--the fluid portion of blood and a medical technology that was just four years old--to replenish bodily liquids that evaporated because of the loss of the protective covering of skin.
"Every war has given us a new medical advance. And penicillin was the great scientific advance of World War II."
"The initial insult with burns is a loss of fluids and patients can die of shock," says Dr. Ken Marshall. "The scientific progress that was made by the two institutions revolutionized fluid management and topical management of burn care forever."
Still, they could not halt the staph infections that kill most burn victims—which prompted the first civilian use of a miracle elixir that was being secretly developed in government-sponsored labs and that ultimately ushered in a new age in therapeutics. Military officials quickly realized this disaster could provide an excellent natural laboratory to test the effectiveness of this drug and see if it could be used to treat the acute traumas of combat in this unfortunate civilian approximation of battlefield conditions. At the time, the very existence of this wondrous medicine—penicillin—was a closely guarded military secret.
From Forgotten Lab Experiment to Wonder Drug
In 1928, Alexander Fleming discovered the curative powers of penicillin, which promised to eradicate infectious pathogens that killed millions every year. But the road to mass producing enough of the highly unstable mold was littered with seemingly unsurmountable obstacles and it remained a forgotten laboratory curiosity for over a decade. But Fleming never gave up and penicillin's eventual rescue from obscurity was a landmark in scientific history.
In 1940, a group at Oxford University, funded in part by the Rockefeller Foundation, isolated enough penicillin to test it on twenty-five mice, which had been infected with lethal doses of streptococci. Its therapeutic effects were miraculous—the untreated mice died within hours, while the treated ones played merrily in their cages, undisturbed. Subsequent tests on a handful of patients, who were brought back from the brink of death, confirmed that penicillin was indeed a wonder drug. But Britain was then being ravaged by the German Luftwaffe during the Blitz, and there were simply no resources to devote to penicillin during the Nazi onslaught.
In June of 1941, two of the Oxford researchers, Howard Florey and Ernst Chain, embarked on a clandestine mission to enlist American aid. Samples of the temperamental mold were stored in their coats. By October, the Roosevelt Administration had recruited four companies—Merck, Squibb, Pfizer and Lederle—to team up in a massive, top-secret development program. Merck, which had more experience with fermentation procedures, swiftly pulled away from the pack and every milligram they produced was zealously hoarded.
After the nightclub fire, the government ordered Merck to dispatch to Boston whatever supplies of penicillin that they could spare and to refine any crude penicillin broth brewing in Merck's fermentation vats. After working in round-the-clock relays over the course of three days, on the evening of December 1st, 1942, a refrigerated truck containing thirty-two liters of injectable penicillin left Merck's Rahway, New Jersey plant. It was accompanied by a convoy of police escorts through four states before arriving in the pre-dawn hours at Massachusetts General Hospital. Dozens of people were rescued from near-certain death in the first public demonstration of the powers of the antibiotic, and the existence of penicillin could no longer be kept secret from inquisitive reporters and an exultant public. The next day, the Boston Globe called it "priceless" and Time magazine dubbed it a "wonder drug."
Within fourteen months, penicillin production escalated exponentially, churning out enough to save the lives of thousands of soldiers, including many from the Normandy invasion. And in October 1945, just weeks after the Japanese surrender ended World War II, Alexander Fleming, Howard Florey and Ernst Chain were awarded the Nobel Prize in medicine. But penicillin didn't just save lives—it helped build some of the most innovative medical and scientific companies in history, including Merck, Pfizer, Glaxo and Sandoz.
"Every war has given us a new medical advance," concludes Marshall. "And penicillin was the great scientific advance of World War II."
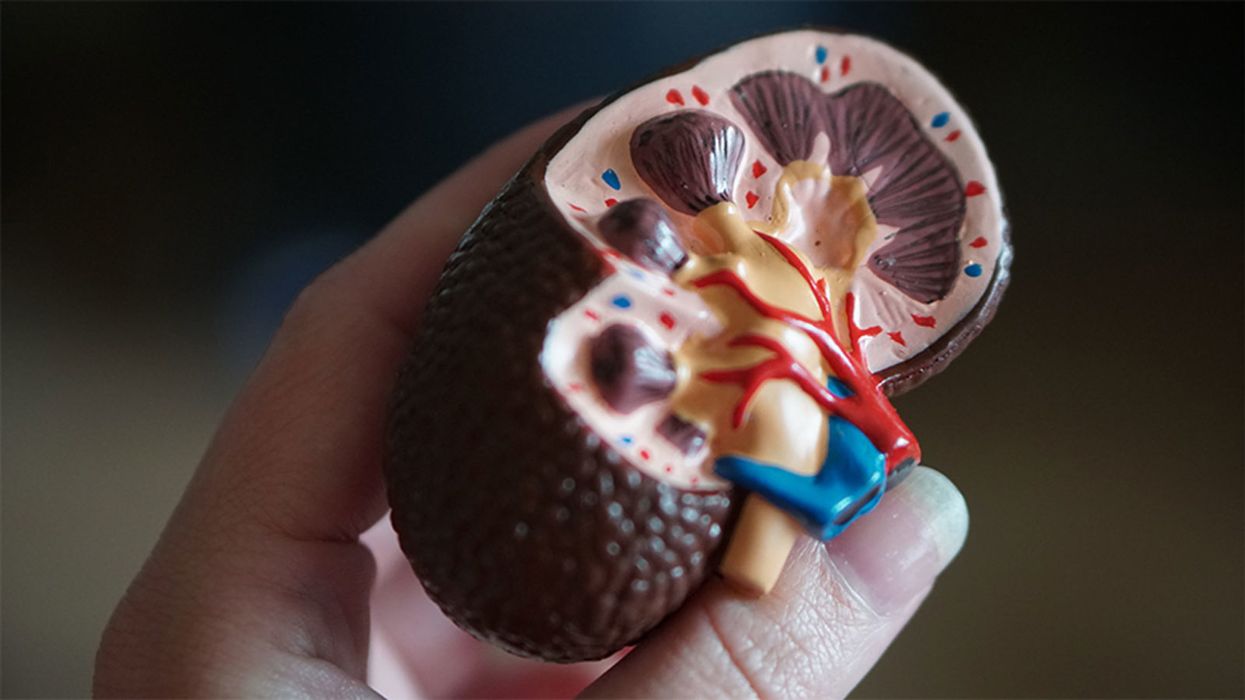
A model of a human kidney.
Science's dream of creating perfect custom organs on demand as soon as a patient needs one is still a long way off. But tiny versions are already serving as useful research tools and stepping stones toward full-fledged replacements.
Although organoids cannot yet replace kidneys, they are invaluable tools for research.
The Lowdown
Australian researchers have grown hundreds of mini human kidneys in the past few years. Known as organoids, they function much like their full-grown counterparts, minus a few features due to a lack of blood supply.
Cultivated in a petri dish, these kidneys are still a shadow of their human counterparts. They grow no larger than one-sixth of an inch in diameter; fully developed organs are up to five inches in length. They contain no more than a few dozen nephrons, the kidney's individual blood-filtering unit, whereas a fully-grown kidney has about 1 million nephrons. And the dish variety live for just a few weeks.
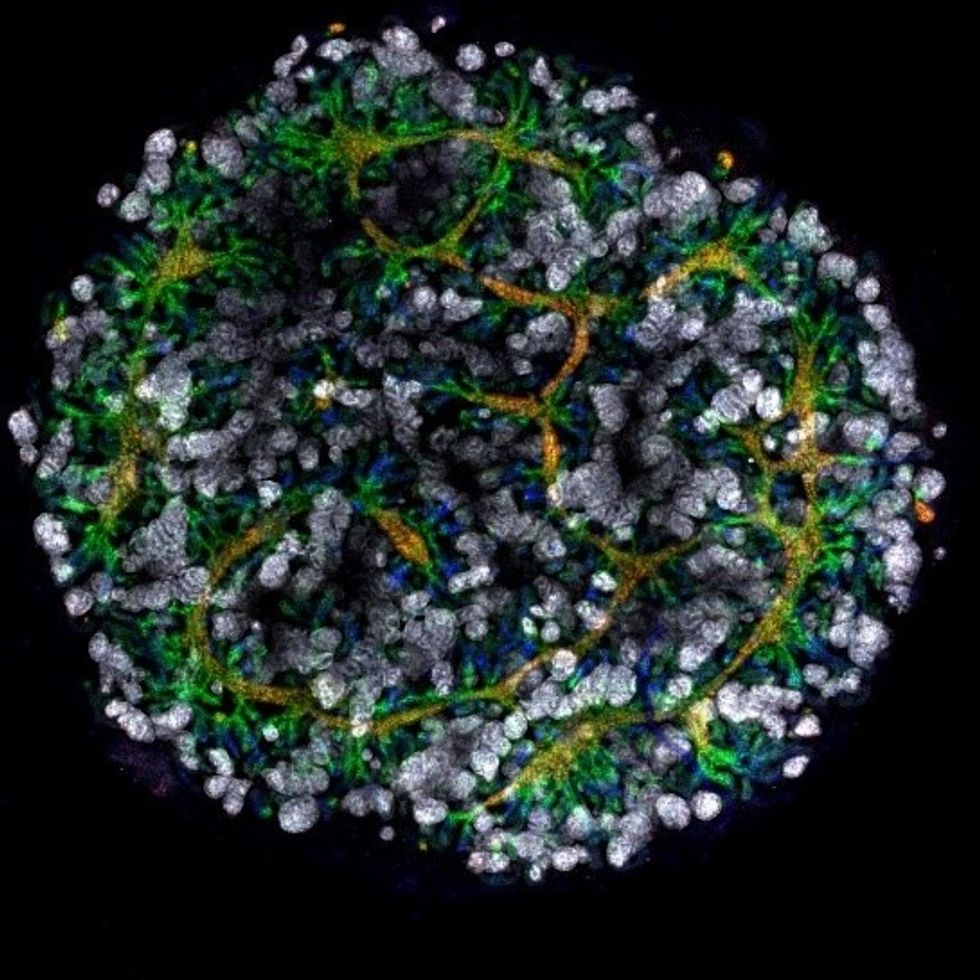
An organoid kidney created by the Murdoch Children's Institute in Melbourne, Australia.
Photo Credit: Shahnaz Khan.
But Melissa Little, head of the kidney research laboratory at the Murdoch Children's Institute in Melbourne, says these organoids are invaluable tools for research. Although renal failure is rare in children, more than half of those who suffer from such a disorder inherited it.
The mini kidneys enable scientists to better understand the progression of such disorders because they can be grown with a patient's specific genetic condition.
Mature stem cells can be extracted from a patient's blood sample and then reprogrammed to become like embryonic cells, able to turn into any type of cell in the body. It's akin to walking back the clock so that the cells regain unlimited potential for development. (The Japanese scientist who pioneered this technique was awarded the Nobel Prize in 2012.) These "induced pluripotent stem cells" can then be chemically coaxed to grow into mini kidneys that have the patient's genetic disorder.
"The (genetic) defects are quite clear in the organoids, and they can be monitored in the dish," Little says. To date, her research team has created organoids from 20 different stem cell lines.
Medication regimens can also be tested on the organoids, allowing specific tailoring for each patient. For now, such testing remains restricted to mice, but Little says it eventually will be done on human organoids so that the results can more accurately reflect how a given patient will respond to particular drugs.
Next Steps
Although these organoids cannot yet replace kidneys, Little says they may plug a huge gap in renal care by assisting in developing new treatments for chronic conditions. Currently, most patients with a serious kidney disorder see their options narrow to dialysis or organ transplantation. The former not only requires multiple sessions a week, but takes a huge toll on patient health.
Ten percent of older patients on dialysis die every year in the U.S. Aside from the physical trauma of organ transplantation, finding a suitable donor outside of a family member can be difficult.
"This is just another great example of the potential of pluripotent stem cells."
Meanwhile, the ongoing creation of organoids is supplying Little and her colleagues with enough information to create larger and more functional organs in the future. According to Little, researchers in the Netherlands, for example, have found that implanting organoids in mice leads to the creation of vascular growth, a potential pathway toward creating bigger and better kidneys.
And while Little acknowledges that creating a fully-formed custom organ is the ultimate goal, the mini organs are an important bridge step.
"This is just another great example of the potential of pluripotent stem cells, and I am just passionate to see it do some good."
Phil Gutis, an Alzheimer's patient who participated in a failed clinical trial, poses with his dog Abe.
Phil Gutis never had a stellar memory, but when he reached his early 50s, it became a problem he could no longer ignore. He had trouble calculating how much to tip after a meal, finding things he had just put on his desk, and understanding simple driving directions.
From 1998-2017, industry sources reported 146 failed attempts at developing Alzheimer's drugs.
So three years ago, at age 54, he answered an ad for a drug trial seeking people experiencing memory issues. He scored so low in the memory testing he was told something was wrong. M.R.I.s and PET scans confirmed that he had early-onset Alzheimer's disease.
Gutis, who is a former New York Times reporter and American Civil Liberties Union spokesman, felt fortunate to get into an advanced clinical trial of a new treatment for Alzheimer's disease. The drug, called aducanumab, had shown promising results in earlier studies.
Four years of data had found that the drug effectively reduced the burden of protein fragments called beta-amyloids, which destroy connections between nerve cells. Amyloid plaques are found in the brains of patients with Alzheimer's disease and are associated with impairments in thinking and memory.
Gutis eagerly participated in the clinical trial and received 35 monthly infusions. "For the first 20 infusions, I did not know whether I was receiving the drug or the placebo," he says. "During the last 15 months, I received aducanumab. But it really didn't matter if I was receiving the drug or the placebo because on March 21, the trial was stopped because [the drug company] Biogen found that the treatments were ineffective."
The news was devastating to the trial participants, but also to the Alzheimer's research community. Earlier this year, another pharmaceutical company, Roche, announced it was discontinuing two of its Alzheimer's clinical trials. From 1998-2017, industry sources reported 146 failed attempts at developing Alzheimer's drugs. There are five prescription drugs approved to treat its symptoms, but a cure remains elusive. The latest failures have left researchers scratching their heads about how to approach attacking the disease.
The failure of aducanumab was also another setback for the estimated 5.8 million people who have Alzheimer's in the United States. Of these, around 5.6 million are older than 65 and 200,000 suffer from the younger-onset form, including Gutis.
Gutis is understandably distraught about the cancellation of the trial. "I really had hopes it would work. So did all the patients."
While drug companies have failed so far, another group is stepping up to expedite the development of a cure: venture philanthropists.
For now, he is exercising every day to keep his blood flowing, which is supposed to delay the progression of the disease, and trying to eat a low-fat diet. "But I know that none of it will make a difference. Alzheimer's is a progressive disease. There are no treatments to delay it, let alone cure it."
But while drug companies have failed so far, another group is stepping up to expedite the development of a cure: venture philanthropists. These are successful titans of industry and dedicated foundations who are donating large sums of money to fill a much-needed void – funding research to look for new biomarkers.
Biomarkers are neurochemical indicators that can be used to detect the presence of a disease and objectively measure its progression. There are currently no validated biomarkers for Alzheimer's, but researchers are actively studying promising candidates. The hope is that they will find a reliable way to identify the disease even before the symptoms of mental decline show up, so that treatments can be directed at a very early stage.
Howard Fillit, Founding Executive Director and Chief Science Officer of the Alzheimer's Drug Discovery Foundation, says, "We need novel biomarkers to diagnose Alzheimer's disease and related dementias. But pharmaceutical companies don't put money into biomarkers research."
One of the venture philanthropists who has recently stepped up to the task is Bill Gates. In January 2018, he announced his father had Alzheimer's disease in an interview on the Today Show with Maria Shriver, whose father Sargent Shriver, died of Alzheimer's disease in 2011. Gates told Ms. Shriver that he had invested $100 million into Alzheimer's research, with $50 million of his donation going to Dementia Discovery Fund, which looks for new cures and treatments.
That August, Gates joined other investors in a new fund called Diagnostics Accelerator. The project aims to supports researchers looking to speed up new ideas for earlier and better diagnosis of the disease.
Gates and other donors committed more than $35 million to help launch it, and this April, Jeff and Mackenzie Bezos joined the coalition, bringing the current program funding to nearly $50 million.
"It makes sense that a challenge this significant would draw the attention of some of the world's leading thinkers."
None of these funders stand to make a profit on their donation, unlike traditional research investments by drug companies. The standard alternatives to such funding have upsides -- and downsides.
As Bill Gates wrote on his blog, "Investments from governments or charitable organizations are fantastic at generating new ideas and cutting-edge research -- but they're not always great at creating usable products, since no one stands to make a profit at the end of the day.
"Venture capital, on the other end of the spectrum, is more likely to develop a test that will reach patients, but its financial model favors projects that will earn big returns for investors. Venture philanthropy splits the difference. It incentivizes a bold, risk-taking approach to research with an end goal of a real product for real patients. If any of the projects backed by Diagnostics Accelerator succeed, our share of the financial windfall goes right back into the fund."
Gutis said he is thankful for any attention given to finding a cure for Alzheimer's.
"Most doctors and scientists will tell you that we're still in the dark ages when it comes to fully understanding how the brain works, let alone figuring out the cause or treatment for Alzheimer's.
"It makes sense that a challenge this significant would draw the attention of some of the world's leading thinkers. I only hope they can be more successful with their entrepreneurial approach to finding a cure than the drug companies have been with their more traditional paths."