How a Deadly Fire Gave Birth to Modern Medicine

The Cocoanut Grove fire in Boston in 1942 tragically claimed 490 lives, but was the catalyst for several important medical advances.
On the evening of November 28, 1942, more than 1,000 revelers from the Boston College-Holy Cross football game jammed into the Cocoanut Grove, Boston's oldest nightclub. When a spark from faulty wiring accidently ignited an artificial palm tree, the packed nightspot, which was only designed to accommodate about 500 people, was quickly engulfed in flames. In the ensuing panic, hundreds of people were trapped inside, with most exit doors locked. Bodies piled up by the only open entrance, jamming the exits, and 490 people ultimately died in the worst fire in the country in forty years.
"People couldn't get out," says Dr. Kenneth Marshall, a retired plastic surgeon in Boston and president of the Cocoanut Grove Memorial Committee. "It was a tragedy of mammoth proportions."
Within a half an hour of the start of the blaze, the Red Cross mobilized more than five hundred volunteers in what one newspaper called a "Rehearsal for Possible Blitz." The mayor of Boston imposed martial law. More than 300 victims—many of whom subsequently died--were taken to Boston City Hospital in one hour, averaging one victim every eleven seconds, while Massachusetts General Hospital admitted 114 victims in two hours. In the hospitals, 220 victims clung precariously to life, in agonizing pain from massive burns, their bodies ravaged by infection.

The scene of the fire.
Boston Public Library
Tragic Losses Prompted Revolutionary Leaps
But there is a silver lining: this horrific disaster prompted dramatic changes in safety regulations to prevent another catastrophe of this magnitude and led to the development of medical techniques that eventually saved millions of lives. It transformed burn care treatment and the use of plasma on burn victims, but most importantly, it introduced to the public a new wonder drug that revolutionized medicine, midwifed the birth of the modern pharmaceutical industry, and nearly doubled life expectancy, from 48 years at the turn of the 20th century to 78 years in the post-World War II years.
The devastating grief of the survivors also led to the first published study of post-traumatic stress disorder by pioneering psychiatrist Alexandra Adler, daughter of famed Viennese psychoanalyst Alfred Adler, who was a student of Freud. Dr. Adler studied the anxiety and depression that followed this catastrophe, according to the New York Times, and "later applied her findings to the treatment World War II veterans."
Dr. Ken Marshall is intimately familiar with the lingering psychological trauma of enduring such a disaster. His mother, an Irish immigrant and a nurse in the surgical wards at Boston City Hospital, was on duty that cold Thanksgiving weekend night, and didn't come home for four days. "For years afterward, she'd wake up screaming in the middle of the night," recalls Dr. Marshall, who was four years old at the time. "Seeing all those bodies lined up in neat rows across the City Hospital's parking lot, still in their evening clothes. It was always on her mind and memories of the horrors plagued her for the rest of her life."
The sheer magnitude of casualties prompted overwhelmed physicians to try experimental new procedures that were later successfully used to treat thousands of battlefield casualties. Instead of cutting off blisters and using dyes and tannic acid to treat burned tissues, which can harden the skin, they applied gauze coated with petroleum jelly. Doctors also refined the formula for using plasma--the fluid portion of blood and a medical technology that was just four years old--to replenish bodily liquids that evaporated because of the loss of the protective covering of skin.
"Every war has given us a new medical advance. And penicillin was the great scientific advance of World War II."
"The initial insult with burns is a loss of fluids and patients can die of shock," says Dr. Ken Marshall. "The scientific progress that was made by the two institutions revolutionized fluid management and topical management of burn care forever."
Still, they could not halt the staph infections that kill most burn victims—which prompted the first civilian use of a miracle elixir that was being secretly developed in government-sponsored labs and that ultimately ushered in a new age in therapeutics. Military officials quickly realized this disaster could provide an excellent natural laboratory to test the effectiveness of this drug and see if it could be used to treat the acute traumas of combat in this unfortunate civilian approximation of battlefield conditions. At the time, the very existence of this wondrous medicine—penicillin—was a closely guarded military secret.
From Forgotten Lab Experiment to Wonder Drug
In 1928, Alexander Fleming discovered the curative powers of penicillin, which promised to eradicate infectious pathogens that killed millions every year. But the road to mass producing enough of the highly unstable mold was littered with seemingly unsurmountable obstacles and it remained a forgotten laboratory curiosity for over a decade. But Fleming never gave up and penicillin's eventual rescue from obscurity was a landmark in scientific history.
In 1940, a group at Oxford University, funded in part by the Rockefeller Foundation, isolated enough penicillin to test it on twenty-five mice, which had been infected with lethal doses of streptococci. Its therapeutic effects were miraculous—the untreated mice died within hours, while the treated ones played merrily in their cages, undisturbed. Subsequent tests on a handful of patients, who were brought back from the brink of death, confirmed that penicillin was indeed a wonder drug. But Britain was then being ravaged by the German Luftwaffe during the Blitz, and there were simply no resources to devote to penicillin during the Nazi onslaught.
In June of 1941, two of the Oxford researchers, Howard Florey and Ernst Chain, embarked on a clandestine mission to enlist American aid. Samples of the temperamental mold were stored in their coats. By October, the Roosevelt Administration had recruited four companies—Merck, Squibb, Pfizer and Lederle—to team up in a massive, top-secret development program. Merck, which had more experience with fermentation procedures, swiftly pulled away from the pack and every milligram they produced was zealously hoarded.
After the nightclub fire, the government ordered Merck to dispatch to Boston whatever supplies of penicillin that they could spare and to refine any crude penicillin broth brewing in Merck's fermentation vats. After working in round-the-clock relays over the course of three days, on the evening of December 1st, 1942, a refrigerated truck containing thirty-two liters of injectable penicillin left Merck's Rahway, New Jersey plant. It was accompanied by a convoy of police escorts through four states before arriving in the pre-dawn hours at Massachusetts General Hospital. Dozens of people were rescued from near-certain death in the first public demonstration of the powers of the antibiotic, and the existence of penicillin could no longer be kept secret from inquisitive reporters and an exultant public. The next day, the Boston Globe called it "priceless" and Time magazine dubbed it a "wonder drug."
Within fourteen months, penicillin production escalated exponentially, churning out enough to save the lives of thousands of soldiers, including many from the Normandy invasion. And in October 1945, just weeks after the Japanese surrender ended World War II, Alexander Fleming, Howard Florey and Ernst Chain were awarded the Nobel Prize in medicine. But penicillin didn't just save lives—it helped build some of the most innovative medical and scientific companies in history, including Merck, Pfizer, Glaxo and Sandoz.
"Every war has given us a new medical advance," concludes Marshall. "And penicillin was the great scientific advance of World War II."
People With This Rare Disease Can Barely Eat Protein. Biotechnology May Change That.
The Brown family at the Grand Tetons (2019). Clockwise from left, Christine, Kevin, Keagan, Connor, and Kellen.
Imagine that the protein in bread, eggs, steak, even beans is not the foundation for a healthy diet, but a poison to your brain. That is the reality for people living with Phenylketonuria, or PKU. This cluster of rare genetic variations affects the ability to digest phenylalanine (Phe), one of the chemical building blocks of protein. The toxins can build up in the brain causing severe mental retardation.
Can a probiotic help digest the troublesome proteins before they can enter the bloodstream and travel to the brain? A Boston area biotech start up, Synlogic, believes it can. Their starting point is an E. coli bacterium that has been used as a probiotic for more than a century. The company then screened thousands of gene variants to identify ones that produced enzymes most efficient at slicing and dicing the target proteins and optimized them further through directed evolution. The results have been encouraging.
But Christine Brown knew none of this when the hospital called saying that standard newborn screening of her son Connor had come back positive for PKU. It was urgent that they visit a special metabolic clinic the next day, which was about a three-hour drive away.
“I was told not to go on the Internet,” Christine recalls, “So when somebody tells you not to go on the Internet, what do you do? Even back in 2005, right.” What she saw were the worst examples of retardation, which was a common outcome from PKU before newborn screening became routine. “We were just in complete shell shock, our whole world just kind of shattered and went into a tail spin.”
“I remember feeding him the night before our clinic visit and almost feeling like I was feeding him poison because I knew that breast milk must have protein in it,” she says.
“Some of my first memories are of asking, ‘Mommy, can I eat this? There were yes foods and no foods.'"
Over the next few days the dedicated staff of the metabolic clinic at the Waisman Center at the University of Wisconsin Madison began to walk she and husband Kevin back from that nightmare. They learned that a simple blood test to screen newborns had been developed in the early 1960s to detect PKU and that the condition could be managed with stringent food restrictions and vigilant monitoring of Phe levels.
Everything in Your Mouth Counts
PKU can be successfully managed with a severely restricted diet. That simple statement is factually true, but practically impossible to follow, as it requires slashing protein consumption by about 90 percent. To compensate for the missing protein, several times a day PKU patients take a medical formula – commonly referred to simply as formula – containing forms of proteins that are digestible to their bodies. Several manufacturers now add vitamins and minerals and offer a variety of formats and tastes to make it more consumer friendly, but that wasn't always the case.
“When I was a kid, it tasted horrible, was the consistency of house paint. I didn't think about it, I just drank it. I didn't like it but you get used to it after a while,” recalls Jeff Wolf, the twang of Appalachia still strong in his voice. Now age 50, he grew up in Ashland, Kentucky and was part of the first wave of persons with PKU who were identified at birth as newborn screening was rolled out across the US. He says the options of taste and consistency have improved tremendously over the years.
Some people with PKU are restricted to as little as 8 grams of protein a day from food. That's a handful of almonds or a single hard-boiled egg; a skimpy 4-ounce hamburger and slice of cheese adds up to half of their weekly protein ration. Anything above that daily allowance is more than the body can handle and toxic levels of Phe begin to accumulate in the brain.
“Some of my first memories are of asking, ‘Mommy, can I eat this? There were yes foods and no foods,’” recalls Les Clark. He has never eaten a hamburger, steak, or ribs, practically a sacrilege for someone raised in Stanton, a small town in northeastern Nebraska, a state where the number of cattle and hogs are several-fold those of people.
His grandmother learned how to make low protein bread, but it looked and tasted different. His mom struggled making birthday cakes. “I learned some bad words at a very young age” as mom struggled applying icing that would pull the cake apart or a slice would collapse into a heap of crumbs, Les recalls.

Les Clark with a birthday cake.
Courtesy Clark
Controlling the diet “is not so bad when you are a baby” because that's all you know, says Jerry Vockley, Director of the Center for Rare Disease Therapy at Children's Hospital of Pittsburgh. “But after a while, as you get older and you start tasting other things and you say, Well, gee, this stuff tastes way better than what you're giving me. What's the deal? It becomes harder to maintain the diet.”
First is the lure of forbidden foods as children venture into the community away from the watchful eyes of parents. The support system weakens further when they leave home for college or work. “Pizza was mighty tasty,” Wolf' says of his first slice.
Vockley estimates that about 90 percent of adults with PKU are off of treatment. Moving might mean finding a new metabolic clinic that treats PKU. A lapse in insurance coverage can be a factor. Finally there is plain fatigue from multiple daily dosing of barely tolerable formula, monitoring protein intake, and simply being different in terms of food restrictions. Most people want to fit in and not be defined by their medical condition.
Jeff Wolf was one of those who dropped out in his twenties and thirties. He stopped going to clinic, monitoring his Phe levels, and counting protein. But the earlier experience of living with PKU never completely left the back of his mind; he listened to his body whenever eating too much protein left him with the “fuzzy brain" of a protein hangover. About a decade ago he reconnected with a metabolic clinic, began taking formula and watching his protein intake. He still may go over his allotment for a single day but he tries to compensate on subsequent days so that his Phe levels come back into balance.

Jeff Wolf on a boat.
Courtesy Wolf
One of the trickiest parts of trying to manage phenylalanine intake is the artificial sweetener aspartame. The chemical is ubiquitous in diet and lite foods and drinks. Gum too, you don't even have to swallow to receive a toxic dose of Phe. Most PKUers say it is easier to simply avoid these products entirely rather than try to count their Phe content.
Treatments
Most rare diseases have no treatment. There are two drugs for PKU that provide some benefit to some portion of patients but those drugs often have their own burdens.
KUVAN® (sapropterin dihydrochloride) is a pill or powder that helps correct a protein folding error so that food proteins can be digested. It is approved for most types of PKU in adults and children one month and older, and often is used along with a protein-restricted diet.
“The problem is that it doesn't work for every [patient's genetic] mutation, and there are hundreds of mutations that have been identified with PKU. Two to three percent of patients will have a very dramatic response and if you're one of those small number of patients, it's great,” says Vockley. “If you have one of the other mutation, chances are pretty good you still are going to end up on a restricted diet.”
PALYNZIQ® (pegvaliase-pqpz) “has the potential to lower the Phe to normal levels, it's a real breakthrough in the field,” says Vockley. “But is a very hard drug to use. Most folks have to take either one or two 2ml injections a day of something that is basically a gel, and some individuals have to take three.”
Many PKUers have reactions at the site of the injection and some develop anaphylaxis, a severe potentially life-threatening allergic reaction that can happen within seconds and can occur at any time, even after long term use. Many patients using Palynziq carry an EpiPen, a self-injection devise containing a form of adrenaline that can reverse some of the symptoms of anaphylaxis.
Then there is the cost. With the Kuvan dosing for an adult, “you're talking between $100,000 and $200,000 a year. And Palynziq is three times that,” says Vockley. Insurance coverage through a private plan or a state program is essential. Some state programs provide generous coverage while others are skimpy. Most large insurance company plans cover the drugs, sometimes with significant copays, but companies that are self-insured are under no legal obligation to provide coverage.
Les Clark found that out the hard way when the company he worked for was sold. The new owner was self-insured and declined to continue covering his drugs. Almost immediately he was out of pocket an additional $1500 a month for formula, and that was with a substantial discount through the manufacturer's patient support program. He says, “If you don't have an insurance policy that will cover the formula, it's completely unaffordable.” He quickly began to look for a new job.
Hope
It's easy to see why PKUers are eager for advances that will make managing their condition more effect, easier, and perhaps more affordable. Synlogic's efforts have drawn their attention and raised hopes.
Just before Thanksgiving Jerry Vockley presented the latest data to a metabolism conference meeting in Australia. There were only 8 patients in this group of a phase 2 trial using the original version of the company's lead E. coli product, SYNB1618, but they were intensely studied. Each was given the probiotic and then a challenge meal. Vockley saw a 40% reduction in Phe absorption and later a 20% reduction in mean fasting Phe levels in the blood. The product was easy to use and tolerate.
The company also presented early results for SYNB1934, a follow on version that further genetically tweaked the E. coli to roughly double the capacity to chop up the target proteins. Synlogic is recruiting patients for studies to determine the best dosing, which they are planning for next year.
“It's an exciting approach,” says Lex Cowsert, Director of Research Development at the National PKU Alliance, a nonprofit that supports the patient, family, and research communities involved with PKU. “Every patient is different, every patient has a different tolerance for the type of therapy that they are willing to pursue,” and if it pans out, it will be a welcome addition, either alone or in combination with other approaches, to living with PKU.
Author's Note: Reporting this story was made possible by generous support from the National Press Foundation and the Fondation Ipsen. Thanks to the people who so generously shared their time and stories in speaking with me.
Are Brain Implants the Future of Treatment for Depression and Anxiety?
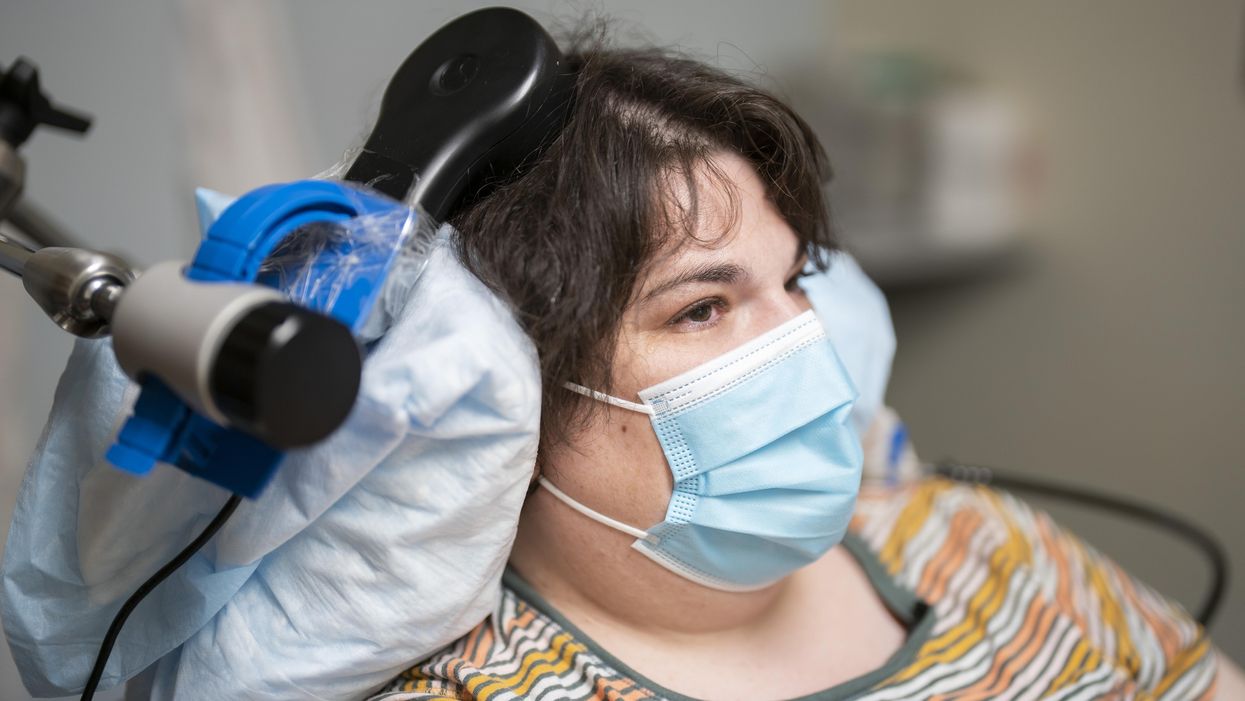
Sarah, clinical trial participant, at an appointment with Katherine Scangos, MD, PhD, at UCSF’s Langley Porter Psychiatric Institute.
When she woke up after a procedure involving drilling small holes in her skull, a woman suffering from chronic depression reported feeling “euphoric”. The holes were made to fit the wires that connected her brain with a matchbox-sized electrical implant; this would deliver up to 300 short-lived electricity bursts per day to specific parts of her brain.
Over a year later, Sarah, 36, says the brain implant has turned her life around. A sense of alertness and energy have replaced suicidal thoughts and feelings of despair, which had persisted despite antidepressants and electroconvulsive therapy. Sarah is the first person to have received a brain implant to treat depression, a breakthrough that happened during an experimental study published recently in Nature Medicine.
“What we did was use deep-brain stimulation (DBS), a technique used in the treatment of epilepsy,” says Andrew Krystal, professor of psychiatry at University of California, San Francisco (UCSF), and one of the study’s researchers. DBS typically involves implanting electrodes into specific areas of the brain to reduce seizures not controlled with medication or to remove the part of the brain that causes the seizures. Instead of choosing and stimulating a single brain site though, the UCSF team took a different approach.
They first used 10 electrodes to map Sarah’s brain activity, a phase that lasted 10 days, during which they developed a neural biomarker, a specific pattern of brain activity that indicated the onset of depression symptoms (in Sarah, this was detected in her amygdala, an almondlike structure located near the base of the brain). But they also saw that delivering a tiny burst of electricity to the patient’s ventral striatum, an area of the brain that sits in the center, above and behind the ears, dramatically improved these symptoms. What they had to do was outfit Sara’s brain with a DBS-device programmed to propagate small waves of electricity to the ventral striatum only when it discerned the pattern.
“We are not trying to take away normal responses to the world. We are just trying to eliminate this one thing, which is depression, which impedes patients’ ability to function and deal with normal stuff.”
“It was a personalized treatment not only in where to stimulate, but when to stimulate,” Krystal says. Sarah’s depression translated to low amounts of energy, loss of pleasure and interest in life, and feelings of sluggishness. Those symptoms went away when scientists stimulated her ventral capsule area. When the same area was manipulated by electricity when Sarah’s symptoms “were not there” though, she was feeling more energetic, but this sudden flush of energy soon gave way to feelings of overstimulation and anxiety. “This is a very tangible illustration of why it's best to simulate only when you need it,” says Krystal.
We have the tendency to lump together depression symptoms, but, in reality, they are quite diverse; some people feel sad and lethargic, others stay up all night; some overeat, others don’t eat at all. “This happens because people have different underlying dysfunctions in different parts of their brain. Our approach is targeting the specific brain circuit that modulates different kinds of symptoms. Simply, where we stimulate depends on the specific set of problems a person has,” Krystal says. Such tailormade brain stimulation for patients with long-term, drug-resistant depression, which would be easy to use at home, could be transformative, the UCSF researcher concludes.
In the U.S., 12.7 percent of the population is on antidepressants. Almost exactly the same percentage of Australians–12.5–take similar drugs every day. With 13 percent of its population being on antidepressants, Iceland is the world’s highest antidepressant consumer. And quite away from Scandinavia, the Southern European country of Portugal is the world’s third strongest market for corresponding medication.
By 2020, nearly 15.5 million people had been consuming antidepressants for a time period exceeding five years. Between 40 and 60 percent of them saw improvements. “For those people, it was absolutely what they needed, whether that was increased serotonin, or increased norepinephrine or increased dopamine, ” says Frank Anderson, a psychiatrist who has been administering antidepressants in his private practice “for a long time”, and author of Transcending Trauma, a book about resolving complex and dissociative trauma.
Yet the UCSF study brings to the mental health field a specificity it has long lacked. “A lot of the traditional medications only really work on six neurotransmitters, when there are over 100 neurotransmitters in the brain,” Anderson says. Drugs are changing the chemistry of a single system in the brain, but brain stimulation is essentially changing the very architecture of the brain, says James Giordano, professor of neurology and biochemistry at Georgetown University Medical Center in Washington and a neuroethicist. It is a far more elegant approach to treating brain disorders, with the potential to prove a lifesaver for the 40 to 50 percent of patients who see no benefits at all with antidepressants, Giordano says. It is neurofeedback, on steroids, adds Anderson. But it comes with certain risks.
Even if the device generating the brain stimulation sits outside the skull and could be easily used at home, the whole process still involves neurosurgery. While the sophistication and precision of brain surgeries has significantly improved over the last years, says Giordano, they always carry risks, such as an allergic reaction to anesthesia, bleeding in the brain, infection at the wound site, blood clots, even coma. Non-invasive brain stimulation (NIBS), a technology currently being developed by the Defense Advanced Research Projects Agency (DARPA), could potentially tackle this. Patients could wear a cap, helmet, or visor that transmits electrical signals from the brain to a computer system and back, in a brain-computer interface that would not need surgery.
“This could counter the implantation of hardware into the brain and body, around which there is also a lot of public hesitance,” says Giordano, who is working on such techniques at DARPA.
Embedding a chip in your head is one of the finest examples of biohacking, an umbrella word for all the practices aimed at hacking one’s body and brain to enhance performance –a citizen do-it-yourself biology. It is also a word charged enough to set off a public backlash. Large segments of the population will simply refuse to allow that level of invasiveness in their heads, says Laura Cabrera, an associate professor of neuroethics at the Center for Neural Engineering, Department of Engineering Science and Mechanics at Penn State University. Cabrera urges caution when it comes to DBS’s potential.
“We've been using it for Parkinson's for over two decades, hoping that now that they get DBS, patients will get off medications. But people have continued taking their drugs, even increasing them,” she says. What the UCSF found is a proof of concept that DBS worked in one depressed person, but there’s a long way ahead until we can confidently say this finding is generalizable to a large group of patients. Besides, as a society, we are not there yet, says Cabrera. “Most people, at least in my research, say they don't want to have things in their brain,” she says. But what could really go wrong if we biohacked our own brains anyway?
In 2014, a man who had received a deep brain implant for a movement disorder started developing an affection for Johnny Cash’s music when he had previously been an avid country music fan. Many protested that the chip had tampered with his personality. Could sparking the brain with electricity generated by a chip outside it put an end to our individuality, messing with our musical preferences, unique quirks, our deeper sense of ego?
“What we found is that when you stimulate a region, you affect people’s moods, their energies,” says Krystal. You are neither changing their personality nor creating creatures of eternal happiness, he says. “’Being on a phone call would generally be a setting that would normally trigger symptoms of depression in me,’” Krystal reports his patient telling him. ‘I now know bad things happen, but am not affected by them in the same way. They don’t trigger the depression.’” Of the research, Krystal continues: “We are not trying to take away normal responses to the world. We are just trying to eliminate this one thing, which is depression, which impedes patients’ ability to function and deal with normal stuff.”
Yet even change itself shouldn't be seen as threatening, especially if the patient had probably desired it in the first place. “The intent of therapy in psychiatric disorders is to change the personality, because a psychiatric disorder by definition is a disorder of personality,” says Cabrera. A person in therapy wants to restore the lost sense of “normal self”. And as for this restoration altering your original taste in music, Cabrera says we are talking about rarities, extremely scarce phenomena that are possible with medication as well.
Maybe it is the allure of dystopian sci-fi films: people have a tendency to worry about dark forces that will spread malice across the world when the line between human and machine has blurred. Such mind-control through DBS would probably require a decent leap of logic with the tools science has--at least to this day. “This would require an understanding of the parameters of brain stimulation we still don't have,” says Cabrera. Still, brain implants are not fully corrupt-proof.
“Hackers could shut off the device or change the parameters of the patient's neurological function enhancing symptoms or creating harmful side-effects,” says Giordano.
There are risks, but also failsafe ways to tackle them, adds Anderson. “Just like medications are not permanent, we could ensure the implants are used for a specific period of time,” he says. And just like people go in for checkups when they are under medication, they could periodically get their personal brain implants checked to see if they have been altered or not, he continues. “It is what my research group refers to as biosecurity by design,” says Giordano. “It is important that we proactively design systems that cannot be corrupted.”
Two weeks after receiving the implant, Sarah scored 14 out of 54 on the Montgomery-Åsberg Depression Rating Scale, a ten-item questionnaire psychiatrists use to measure the severity of depressive episodes. She had initially scored 36. Today she scores under 10. She would have had to wait between four and eight weeks to see positive results had she taken the antidepressant road, says Krystal.
He and his team have enrolled two other patients in the trials and hope to add nine more. They already have some preliminary evidence that there's another place that works better in the brain of another patient, because that specific patient had been experiencing more anxiety as opposed to despondency. Almost certainly, we will have different biomarkers for different people, and brain stimulation will be tailored to a person’s unique situation, says Krystal. “Each brain is different, just like each face is different.”