People With This Rare Disease Can Barely Eat Protein. Biotechnology May Change That.

The Brown family at the Grand Tetons (2019). Clockwise from left, Christine, Kevin, Keagan, Connor, and Kellen.
Imagine that the protein in bread, eggs, steak, even beans is not the foundation for a healthy diet, but a poison to your brain. That is the reality for people living with Phenylketonuria, or PKU. This cluster of rare genetic variations affects the ability to digest phenylalanine (Phe), one of the chemical building blocks of protein. The toxins can build up in the brain causing severe mental retardation.
Can a probiotic help digest the troublesome proteins before they can enter the bloodstream and travel to the brain? A Boston area biotech start up, Synlogic, believes it can. Their starting point is an E. coli bacterium that has been used as a probiotic for more than a century. The company then screened thousands of gene variants to identify ones that produced enzymes most efficient at slicing and dicing the target proteins and optimized them further through directed evolution. The results have been encouraging.
But Christine Brown knew none of this when the hospital called saying that standard newborn screening of her son Connor had come back positive for PKU. It was urgent that they visit a special metabolic clinic the next day, which was about a three-hour drive away.
“I was told not to go on the Internet,” Christine recalls, “So when somebody tells you not to go on the Internet, what do you do? Even back in 2005, right.” What she saw were the worst examples of retardation, which was a common outcome from PKU before newborn screening became routine. “We were just in complete shell shock, our whole world just kind of shattered and went into a tail spin.”
“I remember feeding him the night before our clinic visit and almost feeling like I was feeding him poison because I knew that breast milk must have protein in it,” she says.
“Some of my first memories are of asking, ‘Mommy, can I eat this? There were yes foods and no foods.'"
Over the next few days the dedicated staff of the metabolic clinic at the Waisman Center at the University of Wisconsin Madison began to walk she and husband Kevin back from that nightmare. They learned that a simple blood test to screen newborns had been developed in the early 1960s to detect PKU and that the condition could be managed with stringent food restrictions and vigilant monitoring of Phe levels.
Everything in Your Mouth Counts
PKU can be successfully managed with a severely restricted diet. That simple statement is factually true, but practically impossible to follow, as it requires slashing protein consumption by about 90 percent. To compensate for the missing protein, several times a day PKU patients take a medical formula – commonly referred to simply as formula – containing forms of proteins that are digestible to their bodies. Several manufacturers now add vitamins and minerals and offer a variety of formats and tastes to make it more consumer friendly, but that wasn't always the case.
“When I was a kid, it tasted horrible, was the consistency of house paint. I didn't think about it, I just drank it. I didn't like it but you get used to it after a while,” recalls Jeff Wolf, the twang of Appalachia still strong in his voice. Now age 50, he grew up in Ashland, Kentucky and was part of the first wave of persons with PKU who were identified at birth as newborn screening was rolled out across the US. He says the options of taste and consistency have improved tremendously over the years.
Some people with PKU are restricted to as little as 8 grams of protein a day from food. That's a handful of almonds or a single hard-boiled egg; a skimpy 4-ounce hamburger and slice of cheese adds up to half of their weekly protein ration. Anything above that daily allowance is more than the body can handle and toxic levels of Phe begin to accumulate in the brain.
“Some of my first memories are of asking, ‘Mommy, can I eat this? There were yes foods and no foods,’” recalls Les Clark. He has never eaten a hamburger, steak, or ribs, practically a sacrilege for someone raised in Stanton, a small town in northeastern Nebraska, a state where the number of cattle and hogs are several-fold those of people.
His grandmother learned how to make low protein bread, but it looked and tasted different. His mom struggled making birthday cakes. “I learned some bad words at a very young age” as mom struggled applying icing that would pull the cake apart or a slice would collapse into a heap of crumbs, Les recalls.

Les Clark with a birthday cake.
Courtesy Clark
Controlling the diet “is not so bad when you are a baby” because that's all you know, says Jerry Vockley, Director of the Center for Rare Disease Therapy at Children's Hospital of Pittsburgh. “But after a while, as you get older and you start tasting other things and you say, Well, gee, this stuff tastes way better than what you're giving me. What's the deal? It becomes harder to maintain the diet.”
First is the lure of forbidden foods as children venture into the community away from the watchful eyes of parents. The support system weakens further when they leave home for college or work. “Pizza was mighty tasty,” Wolf' says of his first slice.
Vockley estimates that about 90 percent of adults with PKU are off of treatment. Moving might mean finding a new metabolic clinic that treats PKU. A lapse in insurance coverage can be a factor. Finally there is plain fatigue from multiple daily dosing of barely tolerable formula, monitoring protein intake, and simply being different in terms of food restrictions. Most people want to fit in and not be defined by their medical condition.
Jeff Wolf was one of those who dropped out in his twenties and thirties. He stopped going to clinic, monitoring his Phe levels, and counting protein. But the earlier experience of living with PKU never completely left the back of his mind; he listened to his body whenever eating too much protein left him with the “fuzzy brain" of a protein hangover. About a decade ago he reconnected with a metabolic clinic, began taking formula and watching his protein intake. He still may go over his allotment for a single day but he tries to compensate on subsequent days so that his Phe levels come back into balance.

Jeff Wolf on a boat.
Courtesy Wolf
One of the trickiest parts of trying to manage phenylalanine intake is the artificial sweetener aspartame. The chemical is ubiquitous in diet and lite foods and drinks. Gum too, you don't even have to swallow to receive a toxic dose of Phe. Most PKUers say it is easier to simply avoid these products entirely rather than try to count their Phe content.
Treatments
Most rare diseases have no treatment. There are two drugs for PKU that provide some benefit to some portion of patients but those drugs often have their own burdens.
KUVAN® (sapropterin dihydrochloride) is a pill or powder that helps correct a protein folding error so that food proteins can be digested. It is approved for most types of PKU in adults and children one month and older, and often is used along with a protein-restricted diet.
“The problem is that it doesn't work for every [patient's genetic] mutation, and there are hundreds of mutations that have been identified with PKU. Two to three percent of patients will have a very dramatic response and if you're one of those small number of patients, it's great,” says Vockley. “If you have one of the other mutation, chances are pretty good you still are going to end up on a restricted diet.”
PALYNZIQ® (pegvaliase-pqpz) “has the potential to lower the Phe to normal levels, it's a real breakthrough in the field,” says Vockley. “But is a very hard drug to use. Most folks have to take either one or two 2ml injections a day of something that is basically a gel, and some individuals have to take three.”
Many PKUers have reactions at the site of the injection and some develop anaphylaxis, a severe potentially life-threatening allergic reaction that can happen within seconds and can occur at any time, even after long term use. Many patients using Palynziq carry an EpiPen, a self-injection devise containing a form of adrenaline that can reverse some of the symptoms of anaphylaxis.
Then there is the cost. With the Kuvan dosing for an adult, “you're talking between $100,000 and $200,000 a year. And Palynziq is three times that,” says Vockley. Insurance coverage through a private plan or a state program is essential. Some state programs provide generous coverage while others are skimpy. Most large insurance company plans cover the drugs, sometimes with significant copays, but companies that are self-insured are under no legal obligation to provide coverage.
Les Clark found that out the hard way when the company he worked for was sold. The new owner was self-insured and declined to continue covering his drugs. Almost immediately he was out of pocket an additional $1500 a month for formula, and that was with a substantial discount through the manufacturer's patient support program. He says, “If you don't have an insurance policy that will cover the formula, it's completely unaffordable.” He quickly began to look for a new job.
Hope
It's easy to see why PKUers are eager for advances that will make managing their condition more effect, easier, and perhaps more affordable. Synlogic's efforts have drawn their attention and raised hopes.
Just before Thanksgiving Jerry Vockley presented the latest data to a metabolism conference meeting in Australia. There were only 8 patients in this group of a phase 2 trial using the original version of the company's lead E. coli product, SYNB1618, but they were intensely studied. Each was given the probiotic and then a challenge meal. Vockley saw a 40% reduction in Phe absorption and later a 20% reduction in mean fasting Phe levels in the blood. The product was easy to use and tolerate.
The company also presented early results for SYNB1934, a follow on version that further genetically tweaked the E. coli to roughly double the capacity to chop up the target proteins. Synlogic is recruiting patients for studies to determine the best dosing, which they are planning for next year.
“It's an exciting approach,” says Lex Cowsert, Director of Research Development at the National PKU Alliance, a nonprofit that supports the patient, family, and research communities involved with PKU. “Every patient is different, every patient has a different tolerance for the type of therapy that they are willing to pursue,” and if it pans out, it will be a welcome addition, either alone or in combination with other approaches, to living with PKU.
Author's Note: Reporting this story was made possible by generous support from the National Press Foundation and the Fondation Ipsen. Thanks to the people who so generously shared their time and stories in speaking with me.
Scientists are making machines, wearable and implantable, to act as kidneys
Recent advancements in engineering mean that the first preclinical trials for an artificial kidney could happen soon.
Like all those whose kidneys have failed, Scott Burton’s life revolves around dialysis. For nearly two decades, Burton has been hooked up (or, since 2020, has hooked himself up at home) to a dialysis machine that performs the job his kidneys normally would. The process is arduous, time-consuming, and expensive. Except for a brief window before his body rejected a kidney transplant, Burton has depended on machines to take the place of his kidneys since he was 12-years-old. His whole life, the 39-year-old says, revolves around dialysis.
“Whenever I try to plan anything, I also have to plan my dialysis,” says Burton says, who works as a freelance videographer and editor. “It’s a full-time job in itself.”
Many of those on dialysis are in line for a kidney transplant that would allow them to trade thrice-weekly dialysis and strict dietary limits for a lifetime of immunosuppressants. Burton’s previous transplant means that his body will likely reject another donated kidney unless it matches perfectly—something he’s not counting on. It’s why he’s enthusiastic about the development of artificial kidneys, small wearable or implantable devices that would do the job of a healthy kidney while giving users like Burton more flexibility for traveling, working, and more.
Still, the devices aren’t ready for testing in humans—yet. But recent advancements in engineering mean that the first preclinical trials for an artificial kidney could happen soon, according to Jonathan Himmelfarb, a nephrologist at the University of Washington.
“It would liberate people with kidney failure,” Himmelfarb says.
An engineering marvel
Compared to the heart or the brain, the kidney doesn’t get as much respect from the medical profession, but its job is far more complex. “It does hundreds of different things,” says UCLA’s Ira Kurtz.
Kurtz would know. He’s worked as a nephrologist for 37 years, devoting his career to helping those with kidney disease. While his colleagues in cardiology and endocrinology have seen major advances in the development of artificial hearts and insulin pumps, little has changed for patients on hemodialysis. The machines remain bulky and require large volumes of a liquid called dialysate to remove toxins from a patient’s blood, along with gallons of purified water. A kidney transplant is the next best thing to someone’s own, functioning organ, but with over 600,000 Americans on dialysis and only about 100,000 kidney transplants each year, most of those in kidney failure are stuck on dialysis.
Part of the lack of progress in artificial kidney design is the sheer complexity of the kidney’s job. Each of the 45 different cell types in the kidney do something different.
Part of the lack of progress in artificial kidney design is the sheer complexity of the kidney’s job. To build an artificial heart, Kurtz says, you basically need to engineer a pump. An artificial pancreas needs to balance blood sugar levels with insulin secretion. While neither of these tasks is simple, they are fairly straightforward. The kidney, on the other hand, does more than get rid of waste products like urea and other toxins. Each of the 45 different cell types in the kidney do something different, helping to regulate electrolytes like sodium, potassium, and phosphorous; maintaining blood pressure and water balance; guiding the body’s hormonal and inflammatory responses; and aiding in the formation of red blood cells.

There's been little progress for patients during Ira Kurtz's 37 years as a nephrologist. Artificial kidneys would change that.
UCLA
Dialysis primarily filters waste, and does so well enough to keep someone alive, but it isn’t a true artificial kidney because it doesn’t perform the kidney’s other jobs, according to Kurtz, such as sensing levels of toxins, wastes, and electrolytes in the blood. Due to the size and water requirements of existing dialysis machines, the equipment isn’t portable. Physicians write a prescription for a certain duration of dialysis and assess how well it’s working with semi-regular blood tests. The process of dialysis itself, however, is conducted blind. Doctors can’t tell how much dialysis a patient needs based on kidney values at the time of treatment, says Meera Harhay, a nephrologist at Drexel University in Philadelphia.
But it’s the impact of dialysis on their day-to-day lives that creates the most problems for patients. Only one-quarter of those on dialysis are able to remain employed (compared to 85% of similar-aged adults), and many report a low quality of life. Having more flexibility in life would make a major different to her patients, Harhay says.
“Almost half their week is taken up by the burden of their treatment. It really eats away at their freedom and their ability to do things that add value to their life,” she says.
Art imitates life
The challenge for artificial kidney designers was how to compress the kidney’s natural functions into a portable, wearable, or implantable device that wouldn’t need constant access to gallons of purified and sterilized water. The other universal challenge they faced was ensuring that any part of the artificial kidney that would come in contact with blood was kept germ-free to prevent infection.
As part of the 2021 KidneyX Prize, a partnership between the U.S. Department of Health and Human Services and the American Society of Nephrology, inventors were challenged to create prototypes for artificial kidneys. Himmelfarb’s team at the University of Washington’s Center for Dialysis Innovation won the prize by focusing on miniaturizing existing technologies to create a portable dialysis machine. The backpack sized AKTIV device (Ambulatory Kidney to Increase Vitality) will recycle dialysate in a closed loop system that removes urea from blood and uses light-based chemical reactions to convert the urea to nitrogen and carbon dioxide, which allows the dialysate to be recirculated.
Himmelfarb says that the AKTIV can be used when at home, work, or traveling, which will give users more flexibility and freedom. “If you had a 30-pound device that you could put in the overhead bins when traveling, you could go visit your grandkids,” he says.
Kurtz’s team at UCLA partnered with the U.S. Kidney Research Corporation and Arkansas University to develop a dialysate-free desktop device (about the size of a small printer) as the first phase of a progression that will he hopes will lead to something small and implantable. Part of the reason for the artificial kidney’s size, Kurtz says, is the number of functions his team are cramming into it. Not only will it filter urea from blood, but it will also use electricity to help regulate electrolyte levels in a process called electrodeionization. Kurtz emphasizes that these additional functions are what makes his design a true artificial kidney instead of just a small dialysis machine.
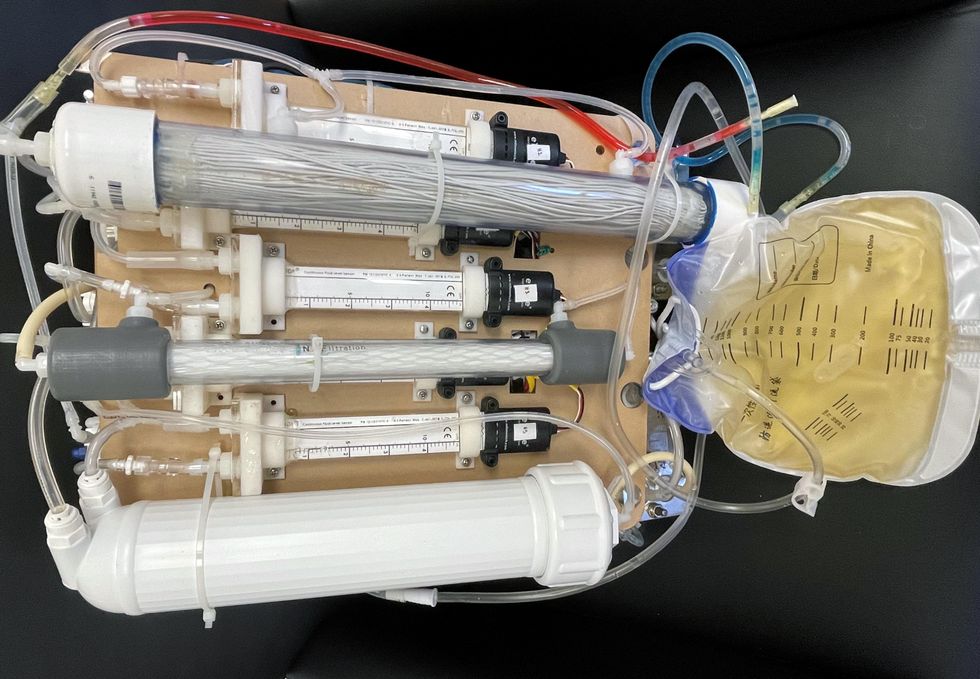
One version of an artificial kidney.
UCLA
“It doesn't have just a static function. It has a bank of sensors that measure chemicals in the blood and feeds that information back to the device,” Kurtz says.
Other startups are getting in on the game. Nephria Bio, a spinout from the South Korean-based EOFlow, is working to develop a wearable dialysis device, akin to an insulin pump, that uses miniature cartridges with nanomaterial filters to clean blood (Harhay is a scientific advisor to Nephria). Ian Welsford, Nephria’s co-founder and CTO, says that the device’s design means that it can also be used to treat acute kidney injuries in resource-limited settings. These potentials have garnered interest and investment in artificial kidneys from the U.S. Department of Defense.
For his part, Burton is most interested in an implantable device, as that would give him the most freedom. Even having a regular outpatient procedure to change batteries or filters would be a minor inconvenience to him.
“Being plugged into a machine, that’s not mimicking life,” he says.
This article was first published by Leaps.org on May 5, 2022.
With this new technology, hospitals and pharmacies could make vaccines and medicines onsite
New research focuses on methods that could change medicine-making worldwide. The scientists propose bursting cells open, removing their DNA and using the cellular gears inside to make therapies.
Most modern biopharmaceutical medicines are produced by workhorse cells—typically bacterial but sometimes mammalian. The cells receive the synthesizing instructions on a snippet of a genetic code, which they incorporate into their DNA. The cellular machinery—ribosomes, RNAs, polymerases, and other compounds—read and use these instructions to build the medicinal molecules, which are harvested and administered to patients.
Although a staple of modern pharma, this process is complex and expensive. One must first insert the DNA instructions into the cells, which they may or may not uptake. One then must grow the cells, keeping them alive and well, so that they produce the required therapeutics, which then must be isolated and purified. To make this at scale requires massive bioreactors and big factories from where the drugs are distributed—and may take a while to arrive where they’re needed. “The pandemic showed us that this method is slow and cumbersome,” says Govind Rao, professor of biochemical engineering who directs the Center for Advanced Sensor Technology at the University of Maryland, Baltimore County (UMBC). “We need better methods that can work faster and can work locally where an outbreak is happening.”
Rao and his team of collaborators, which spans multiple research institutions, believe they have a better approach that may change medicine-making worldwide. They suggest forgoing the concept of using living cells as medicine-producers. Instead, they propose breaking the cells and using the remaining cellular gears for assembling the therapeutic compounds. Instead of inserting the DNA into living cells, the team burst them open, and removed their DNA altogether. Yet, the residual molecular machinery of ribosomes, polymerases and other cogwheels still functioned the way it would in a cell. “Now if you drop your DNA drug-making instructions into that soup, this machinery starts making what you need,” Rao explains. “And because you're no longer worrying about living cells, it becomes much simpler and more efficient.” The collaborators detail their cell-free protein synthesis or CFPS method in their recent paper published in preprint BioAxiv.
While CFPS does not use living cells, it still needs the basic building blocks to assemble proteins from—such as amino acids, nucleotides and certain types of enzymes. These are regularly added into this “soup” to keep the molecular factory chugging. “We just mix everything in as a batch and we let it integrate,” says James Robert Swartz, professor of chemical engineering and bioengineering at Stanford University and co-author of the paper. “And we make sure that we provide enough oxygen.” Rao likens the process to making milk from milk powder.
For a variety of reasons—from the field’s general inertia to regulatory approval hurdles—the method hasn’t become mainstream. The pandemic rekindled interest in medicines that can be made quickly and easily, so it drew more attention to the technology.
The idea of a cell-free protein synthesis is older than one might think. Swartz first experimented with it around 1997, when he was a chemical engineer at Genentech. While working on engineering bacteria to make pharmaceuticals, he discovered that there was a limit to what E. coli cells, the workhorse darling of pharma, could do. For example, it couldn’t grow and properly fold some complex proteins. “We tried many genetic engineering approaches, many fermentation, development, and environmental control approaches,” Swartz recalls—to no avail.
“The organism had its own agenda,” he quips. “And because everything was happening within the organism, we just couldn't really change those conditions very easily. Some of them we couldn’t change at all—we didn’t have control.”
It was out of frustration with the defiant bacteria that a new idea took hold. Could the cells be opened instead, so that the protein-forming reactions could be influenced more easily? “Obviously, we’d lose the ability for them to reproduce,” Swartz says. But that also meant that they no longer needed to keep the cells alive and could focus on making the specific reactions happen. “We could take the catalysts, the enzymes, and the more complex catalysts and activate them, make them work together, much as they would in a living cell, but the way we wanted.”
In 1998, Swartz joined Stanford, and began perfecting the biochemistry of the cell-free method, identifying the reactions he wanted to foster and stopping those he didn’t want. He managed to make the idea work, but for a variety of reasons—from the field’s general inertia to regulatory approval hurdles—the method hasn’t become mainstream. The pandemic rekindled interest in medicines that can be made quickly and easily, so it drew more attention to the technology. For their BioArxiv paper, the team tested the method by growing a specific antiviral protein called griffithsin.
First identified by Barry O’Keefe at National Cancer Institute over a decade ago, griffithsin is an antiviral known to interfere with many viruses’ ability to enter cells—including HIV, SARS, SARS-CoV-2, MERS and others. Originally isolated from the red algae Griffithsia, it works differently from antibodies and antibody cocktails.
Most antiviral medicines tend to target the specific receptors that viruses use to gain entry to the cells they infect. For example, SARS-CoV-2 uses the infamous spike protein to latch onto the ACE2 receptor of mammalian cells. The antibodies or other antiviral molecules stick to the spike protein, shutting off its ability to cling onto the ACE2 receptors. Unfortunately, the spike proteins mutate very often, so the medicines lose their potency. On the contrary, griffithsin has the ability to cling to the different parts of viral shells called capsids—namely to the molecules of mannose, a type of sugar. That extra stuff, glued all around the capsid like dead weight, makes it impossible for the virus to squeeze into the cell.
“Every time we have a vaccine or an antibody against a specific SARS-CoV-2 strain, that strain then mutates and so you lose efficacy,” Rao explains. “But griffithsin molecules glom onto the viral capsid, so the capsid essentially becomes a sticky mess and can’t enter the cell.” Mannose molecules also don’t mutate as easily as viruses’ receptors, so griffithsin-based antivirals do not have to be constantly updated. And because mannose molecules are found on many viruses’ capsids, it makes griffithsin “a universal neutralizer,” Rao explains.
“When griffithsin was discovered, we recognized that it held a lot of promise as a potential antiviral agent,” O’Keefe says. In 2010, he published a paper about griffithsin efficacy in neutralizing viruses of the corona family—after the first SARS outbreak in the early 2000s, the scientific community was interested in such antivirals. Yet, griffithsin is still not available as an off-the-shelf product. So during the Covid pandemic, the team experimented with synthesizing griffithsin using the cell-free production method. They were able to generate potent griffithsin in less than 24 hours without having to grow living cells.
The antiviral protein isn't the only type of medicine that can be made cell-free. The proteins needed for vaccine production could also be made the same way. “Such portable, on-demand drug manufacturing platforms can produce antiviral proteins within hours, making them ideal for combating future pandemics,” Rao says. “We would be able to stop the pandemic before it spreads.”
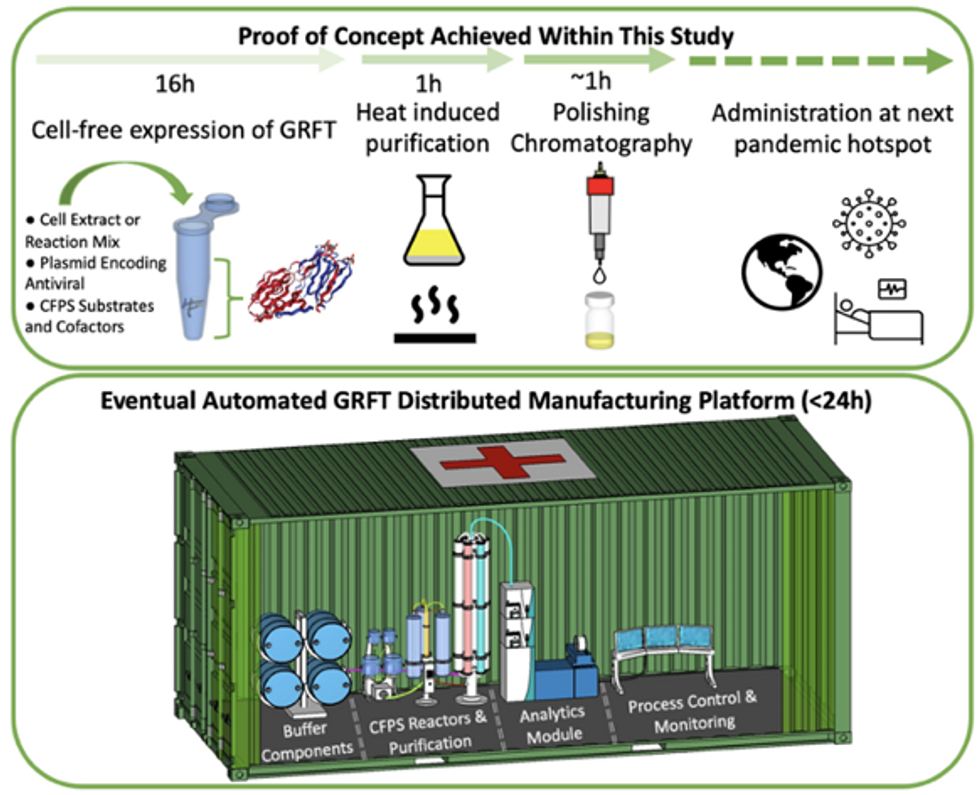
Top: Describes the process used in the study. Bottom: Describes how the new medicines and vaccines could be made at the site of a future viral outbreak.
Image courtesy of Rao and team, sourced from An approach to rapid distributed manufacturing of broad spectrumanti-viral griffithsin using cell-free systems to mitigate pandemics.
Rao’s idea is to perfect the technology to the point that any hospital or pharmacy can load up the media containing molecular factories, mix up the required amino acids, nucleotides and enzymes, and harvest the meds within hours. That will allow making medicines onsite and on demand. “That would be a self-contained production unit, so that you could just ship the production wherever the pandemic is breaking out,” says Swartz.
These units and the meds they produce, will, of course, have to undergo rigorous testing. “The biggest hurdles will be validating these against conventional technology,” Rao says. The biotech industry is risk-averse and prefers the familiar methods. But if this approach works, it may go beyond emergency situations and revolutionize the medicine-making paradigm even outside hospitals and pharmacies. Rao hopes that someday the method might become so mainstream that people may be able to buy and operate such reactors at home. “You can imagine a diabetic patient making insulin that way, or some other drugs,” Rao says. It would work not unlike making baby formula from the mere white powder. Just add water—and some oxygen, too.
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.