Researchers Are Testing a New Stem Cell Therapy in the Hopes of Saving Millions from Blindness

NIH researchers in Kapil Bharti's lab work toward the development of induced pluripotent stem cells to treat dry age-related macular degeneration.
Of all the infirmities of old age, failing sight is among the cruelest. It can mean the end not only of independence, but of a whole spectrum of joys—from gazing at a sunset or a grandchild's face to reading a novel or watching TV.
The Phase 1 trial will likely run through 2022, followed by a larger Phase 2 trial that could last another two or three years.
The leading cause of vision loss in people over 55 is age-related macular degeneration, or AMD, which afflicts an estimated 11 million Americans. As photoreceptors in the macula (the central part of the retina) die off, patients experience increasingly severe blurring, dimming, distortions, and blank spots in one or both eyes.
The disorder comes in two varieties, "wet" and "dry," both driven by a complex interaction of genetic, environmental, and lifestyle factors. It begins when deposits of cellular debris accumulate beneath the retinal pigment epithelium (RPE)—a layer of cells that nourish and remove waste products from the photoreceptors above them. In wet AMD, this process triggers the growth of abnormal, leaky blood vessels that damage the photoreceptors. In dry AMD, which accounts for 80 to 90 percent of cases, RPE cells atrophy, causing photoreceptors to wither away. Wet AMD can be controlled in about a quarter of patients, usually by injections of medication into the eye. For dry AMD, no effective remedy exists.
Stem Cells: Promise and Perils
Over the past decade, stem cell therapy has been widely touted as a potential treatment for AMD. The idea is to augment a patient's ailing RPE cells with healthy ones grown in the lab. A few small clinical trials have shown promising results. In a study published in 2018, for example, a University of Southern California team cultivated RPE tissue from embryonic stem cells on a plastic matrix and transplanted it into the retinas of four patients with advanced dry AMD. Because the trial was designed to test safety rather than efficacy, lead researcher Amir Kashani told a reporter, "we didn't expect that replacing RPE cells would return a significant amount of vision." Yet acuity improved substantially in one recipient, and the others regained their lost ability to focus on an object.
Therapies based on embryonic stem cells, however, have two serious drawbacks: Using fetal cell lines raises ethical issues, and such treatments require the patient to take immunosuppressant drugs (which can cause health problems of their own) to prevent rejection. That's why some experts favor a different approach—one based on induced pluripotent stem cells (iPSCs). Such cells, first produced in 2006, are made by returning adult cells to an undifferentiated state, and then using chemicals to reprogram them as desired. Treatments grown from a patient's own tissues could sidestep both hurdles associated with embryonic cells.
At least hypothetically. Today, the only stem cell therapies approved by the U.S. Food and Drug Administration (FDA) are umbilical cord-derived products for various blood and immune disorders. Although scientists are probing the use of embryonic stem cells or iPSCs for conditions ranging from diabetes to Parkinson's disease, such applications remain experimental—or fraudulent, as a growing number of patients treated at unlicensed "stem cell clinics" have painfully learned. (Some have gone blind after receiving bogus AMD therapies at those facilities.)
Last December, researchers at the National Eye Institute in Bethesda, Maryland, began enrolling patients with dry AMD in the country's first clinical trial using tissue grown from the patients' own stem cells. Led by biologist Kapil Bharti, the team intends to implant custom-made RPE cells in 12 recipients. If the effort pans out, it could someday save the sight of countless oldsters.
That, however, is what's technically referred to as a very big "if."
The First Steps
Bharti's trial is not the first in the world to use patient-derived iPSCs to treat age-related macular degeneration. In 2013, Japanese researchers implanted such cells into the eyes of a 77-year-old woman with wet AMD; after a year, her vision had stabilized, and she no longer needed injections to keep abnormal blood vessels from forming. A second patient was scheduled for surgery—but the procedure was canceled after the lab-grown RPE cells showed signs of worrisome mutations. That incident illustrates one potential problem with using stem cells: Under some circumstances, the cells or the tissue they form could turn cancerous.
"The knowledge and expertise we're gaining can be applied to many other iPSC-based therapies."
Bharti and his colleagues have gone to great lengths to avoid such outcomes. "Our process is significantly different," he told me in a phone interview. His team begins with patients' blood stem cells, which appear to be more genomically stable than the skin cells that the Japanese group used. After converting the blood cells to RPE stem cells, his team cultures them in a single layer on a biodegradable scaffold, which helps them grow in an orderly manner. "We think this material gives us a big advantage," Bharti says. The team uses a machine-learning algorithm to identify optimal cell structure and ensure quality control.
It takes about six months for a patch of iPSCs to become viable RPE cells. When they're ready, a surgeon uses a specially-designed tool to insert the tiny structure into the retina. Within days, the scaffold melts away, enabling the transplanted RPE cells to integrate fully into their new environment. Bharti's team initially tested their method on rats and pigs with eye damage mimicking AMD. The study, published in January 2019 in Science Translational Medicine, found that at ten weeks, the implanted RPE cells continued to function normally and protected neighboring photoreceptors from further deterioration. No trace of mutagenesis appeared.
Encouraged by these results, Bharti began recruiting human subjects. The Phase 1 trial will likely run through 2022, followed by a larger Phase 2 trial that could last another two or three years. FDA approval would require an even larger Phase 3 trial, with a decision expected sometime between 2025 and 2028—that is, if nothing untoward happens before then. One unknown (among many) is whether implanted cells can thrive indefinitely under the biochemically hostile conditions of an eye with AMD.
"Most people don't have a sense of just how long it takes to get something like this to work, and how many failures—even disasters—there are along the way," says Marco Zarbin, professor and chair of Ophthalmology and visual science at Rutgers New Jersey Medical School and co-editor of the book Cell-Based Therapy for Degenerative Retinal Diseases. "The first kidney transplant was done in 1933. But the first successful kidney transplant was in 1954. That gives you a sense of the time frame. We're really taking the very first steps in this direction."
Looking Ahead
Even if Bharti's method proves safe and effective, there's the question of its practicality. "My sense is that using induced pluripotent stem cells to treat the patient from whom they're derived is a very expensive undertaking," Zarbin observes. "So you'd have to have a very dramatic clinical benefit to justify that cost."
Bharti concedes that the price of iPSC therapy is likely to be high, given that each "dose" is formulated for a single individual, requires months to manufacture, and must be administered via microsurgery. Still, he expects economies of scale and production to emerge with time. "We're working on automating several steps of the process," he explains. "When that kicks in, a technician will be able to make products for 10 or 20 people at once, so the cost will drop proportionately."
Meanwhile, other researchers are pressing ahead with therapies for AMD using embryonic stem cells, which could be mass-produced to treat any patient who needs them. But should that approach eventually win FDA approval, Bharti believes there will still be room for a technique that requires neither fetal cell lines nor immunosuppression.
And not only for eye ailments. "The knowledge and expertise we're gaining can be applied to many other iPSC-based therapies," says the scientist, who is currently consulting with several companies that are developing such treatments. "I'm hopeful that we can leverage these approaches for a wide range of applications, whether it's for vision or across the body."
NEI launches iPS cell therapy trial for dry AMD
How Excessive Regulation Helped Ignite COVID-19's Rampant Spread
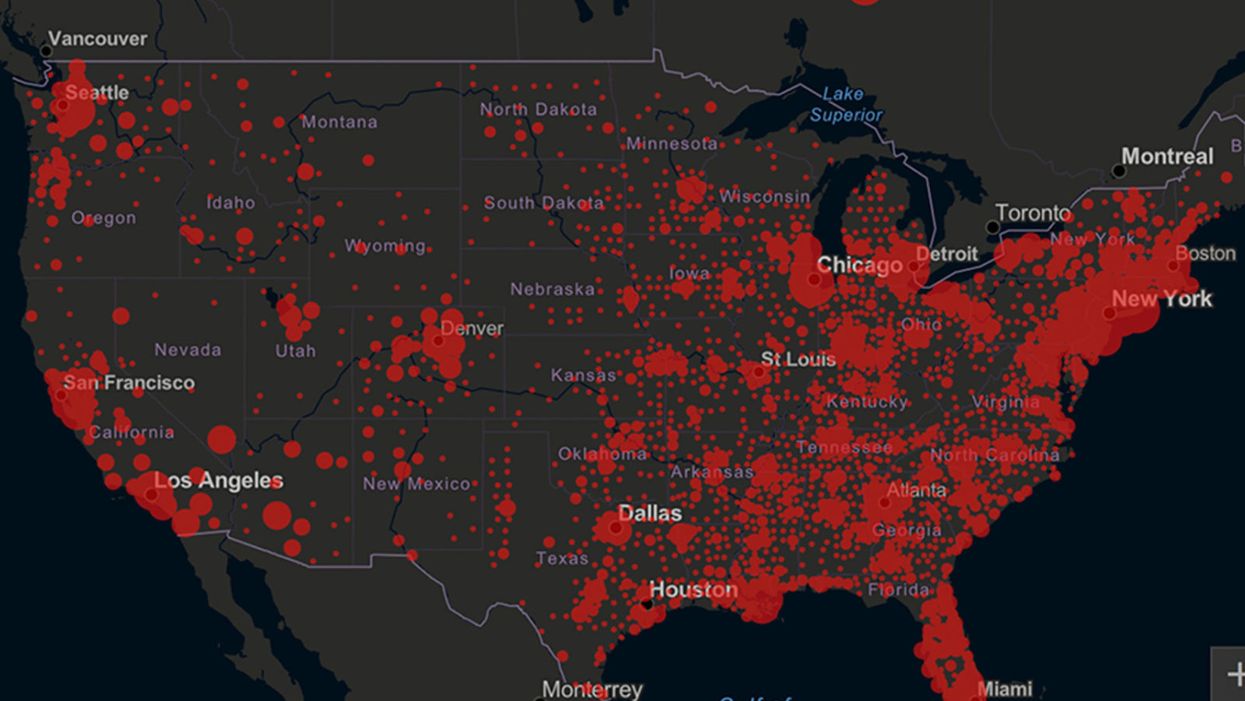
Screenshot of an interactive map of coronavirus cases across the United States, current as of 1:45 p.m. Pacific time on Tuesday, March 31st. Full map accessible at https://coronavirus.jhu.edu/map.html
When historians of the future look back at the 2020 pandemic, the heroic work of Helen Y. Chu, a flu researcher at the University of Washington, will be worthy of recognition.
Chu's team bravely defied the order and conducted the testing anyway.
In late January, Chu was testing nasal swabs for the Seattle Flu Study to monitor influenza spread when she learned of the first case of COVID-19 in Washington state. She deemed it a pressing public health matter to document if and how the illness was spreading locally, so that early containment efforts could succeed. So she sought regulatory approval to adapt the Flu Study to test for the coronavirus, but the federal government denied the request because the original project was funded to study only influenza.
Aware of the urgency, Chu's team bravely defied the order and conducted the testing anyway. Soon they identified a local case in a teenager without any travel history, followed by others. Still, the government tried to shutter their efforts until the outbreak grew dangerous enough to command attention.
Needless testing delays, prompted by excessive regulatory interference, eliminated any chances of curbing the pandemic at its initial stages. Even after Chu went out on a limb to sound alarms, a heavy-handed bureaucracy crushed the nation's ability to roll out early and widespread testing across the country. The Centers for Disease Control and Prevention infamously blundered its own test, while also impeding state and private labs from coming on board, fueling a massive shortage.
The long holdup created "a backlog of testing that needed to be done," says Amesh Adalja, an infectious disease specialist who is a senior scholar at the Johns Hopkins University Center for Health Security.
In a public health crisis, "the ideal situation" would allow the government's test to be "supplanted by private laboratories" without such "a lag in that transition," Adalja says. Only after the eventual release of CDC's test could private industry "begin in earnest" to develop its own versions under the Food and Drug Administration's emergency use authorization.
In a statement, CDC acknowledged that "this process has not gone as smoothly as we would have liked, but there is currently no backlog for testing at CDC."
Now, universities and corporations are in a race against time, playing catch up as the virus continues its relentless spread, also afflicting many health care workers on the front lines.
"Home-testing accessibility is key to preventing further spread of the COVID-19 pandemic."
Hospitals are attempting to add the novel coronavirus to the testing panel of their existent diagnostic machines, which would reduce the results processing time from 48 hours to as little as four hours. Meanwhile, at least four companies announced plans to deliver at-home collection tests to help meet the demand – before a startling injunction by the FDA halted their plans.
Everlywell, an Austin, Texas-based digital health company, had been set to launch online sales of at-home collection kits directly to consumers last week. Scaling up in a matter of days to an initial supply of 30,000 tests, Everlywell collaborated with multiple laboratories where consumers could ship their nasal swab samples overnight, projecting capacity to screen a quarter-million individuals on a weekly basis, says Frank Ong, chief medical and scientific officer.
Secure digital results would have been available online within 48 hours of a sample's arrival at the lab, as well as a telehealth consultation with an independent, board-certified doctor if someone tested positive, for an inclusive $135 cost. The test has a less than 3 percent false-negative rate, Ong says, and in the event of an inadequate self-swab, the lab would not report a conclusive finding. "Home-testing accessibility," he says, "is key to preventing further spread of the COVID-19 pandemic."
But on March 20, the FDA announced restrictions on home collection tests due to concerns about accuracy. The agency did note "the public health value in expanding the availability of COVID-19 testing through safe and accurate tests that may include home collection," while adding that "we are actively working with test developers in this space."
After the restrictions were announced, Everlywell decided to allocate its initial supply of COVID-19 collection kits to hospitals, clinics, nursing homes, and other qualifying health care companies that can commit to no-cost screening of frontline workers and high-risk symptomatic patients. For now, no consumers can order a home-collection test.
"Losing two months is close to disastrous, and that's what we did."
Currently, the U.S. has ramped up to testing an estimated 100,000 people a day, according to Stat News. But 150,000 or more Americans should be tested every day, says Ashish Jha, professor and director of the Harvard Global Health Institute. Due to the dearth of tests, many sick people who suspect they are infected still cannot get confirmation unless they need to be hospitalized.
To give a concrete sense of how far behind we are in testing, consider Palm Beach County, Fla. The state's only drive-thru test center just opened there, requiring an appointment. The center aims to test 750 people per day, but more than 330,000 people have already called to try to book a slot.
"This is such a rapidly moving infection that losing a few days is bad, and losing a couple of weeks is terrible," says Jha, a practicing general internist. "Losing two months is close to disastrous, and that's what we did."
At this point, it will take a long time to fully ramp up. "We are blindfolded," he adds, "and I'd like to take the blindfolds off so we can fight this battle with our eyes wide open."
Better late than never: Yesterday, FDA Commissioner Stephen Hahn said in a statement that the agency has worked with more than 230 test developers and has approved 20 tests since January. An especially notable one was authorized last Friday – 67 days since the country's first known case in Washington state. It's a rapid point-of-care test from medical-device firm Abbott that provides positive results in five minutes and negative results in 13 minutes. Abbott will send 50,000 tests a day to urgent care settings. The first tests are expected to ship tomorrow.
Your Privacy vs. the Public's Health: High-Tech Tracking to Fight COVID-19 Evokes Orwell
Governments around the world are using technology to track their citizens to contain COVID-19.
The COVID-19 pandemic has placed public health and personal privacy on a collision course, as smartphone technology has completely rewritten the book on contact tracing.
It's not surprising that an autocratic regime like China would adopt such measures, but democracies such as Israel have taken a similar path.
The gold standard – patient interviews and detective work – had been in place for more than a century. It's been all but replaced by GPS data in smartphones, which allows contact tracing to occur not only virtually in real time, but with vastly more precision.
China has gone the furthest in using such tech to monitor and prevent the spread of the coronavirus. It developed an app called Health Code to determine which of its citizens are infected or at risk of becoming infected. It has assigned each individual a color code – red, yellow or green – and restricts their movement depending on their assignment. It has also leveraged its millions of public video cameras in conjunction with facial recognition tech to identify people in public who are not wearing masks.
It's not surprising that an autocratic regime like China would adopt such measures, but democracies such as Israel have taken a similar path. The national security agency Shin Bet this week began analyzing all personal cellphone data under emergency measures approved by the government. It texts individuals when it's determined they had been in contact with someone who had the coronavirus. In Spain and China, police have sent drones aloft searching for people violating stay-at-home orders. Commands to disperse can be issued through audio systems built into the aircraft. In the U.S., efforts are underway to lift federal restrictions on drones so that police can use them to prevent people from gathering.
The chief executive of a drone manufacturer in the U.S. aptly summed up the situation in an interview with the Financial Times: "It seems a little Orwellian, but this could save lives."
Epidemics and how they're surveilled often pose thorny dilemmas, according to Craig Klugman, a bioethicist and professor of health sciences at DePaul University in Chicago. "There's always a moral issue to contact tracing," he said, adding that the issue doesn't change by nation, only in the way it's resolved.
"Once certain privacy barriers have been breached, it can be difficult to roll them back again."
In China, there's little to no expectation for privacy, so their decision to take the most extreme measures makes sense to Klugman. "In China, the community comes first. In the U.S., individual rights come first," he said.
As the U.S. has scrambled to develop testing kits and manufacture ventilators to identify potential patients and treat them, individual rights have mostly not received any scrutiny. However, that could change in the coming weeks.
The American approach is also leaning toward using smartphone apps, but in a way that may preserve the privacy of users. Researchers at MIT have released a prototype known as Private Kit: Safe Paths. Patients diagnosed with the coronavirus can use the app to disclose their location trail for the prior 28 days to other users without releasing their specific identity. They also have the option of sharing the data with public health officials. But such an app would only be effective if there is a significant number of users.
Singapore is offering a similar app to its citizens known as TraceTogether, which uses both GPS and Bluetooth pings among users to trace potential encounters. It's being offered on a voluntary basis.
The Electronic Frontier Foundation, the leading nonprofit organization defending civil liberties in the digital world, said it is monitoring how these apps are developed and deployed. "Governments around the world are demanding new dragnet location surveillance powers to contain the COVID-19 outbreak," it said in a statement. "But before the public allows their governments to implement such systems, governments must explain to the public how these systems would be effective in stopping the spread of COVID-19. There's no questioning the need for far-reaching public health measures to meet this urgent challenge, but those measures must be scientifically rigorous, and based on the expertise of public health professionals."
Andrew Geronimo, director of the intellectual property venture clinic at the Case Western University School of Law, said that the U.S. government is currently in talks with Facebook, Google and other tech companies about using deidentified location data from smartphones to better monitor the progress of the outbreak. He was hesitant to endorse such a step.
"These companies may say that all of this data is anonymized," he said, "but studies have shown that it is difficult to fully anonymize data sets that contain so much information about us."
Beyond the technical issues, social attitudes may mount another challenge. Epic events such as 9/11 tend to loosen vigilance toward protecting privacy, according to Klugman and Geronimo. And as more people are sickened and hospitalized in the U.S. with COVID-19, Klugman believes more Americans will be willing to allow themselves to be tracked. "If that happens, there needs to be a time limitation," he said.
However, even if time limits are put in place, Geronimo believes it would lead to an even greater rollback of privacy during the next crisis.
"Once certain privacy barriers have been breached, it can be difficult to roll them back again," he warned. "And the prior incidents could always be used as a precedent – or as proof of concept."