Researchers Are Testing a New Stem Cell Therapy in the Hopes of Saving Millions from Blindness

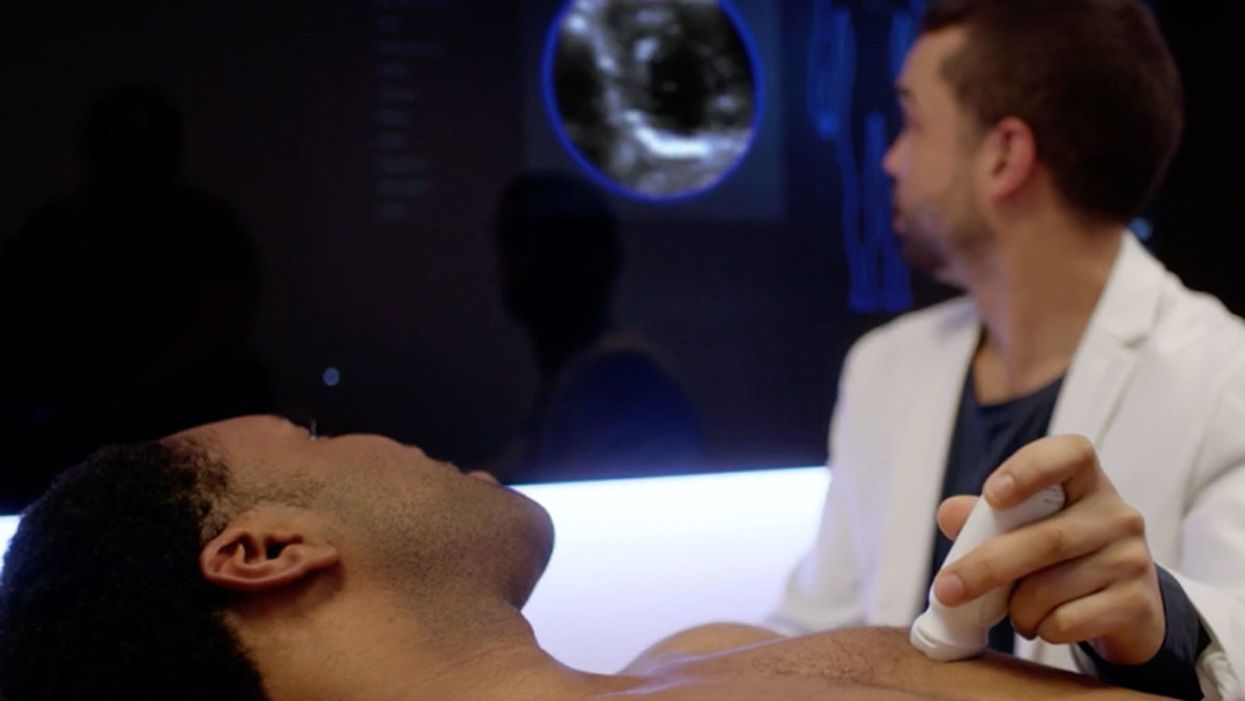
NIH researchers in Kapil Bharti's lab work toward the development of induced pluripotent stem cells to treat dry age-related macular degeneration.
Of all the infirmities of old age, failing sight is among the cruelest. It can mean the end not only of independence, but of a whole spectrum of joys—from gazing at a sunset or a grandchild's face to reading a novel or watching TV.
The Phase 1 trial will likely run through 2022, followed by a larger Phase 2 trial that could last another two or three years.
The leading cause of vision loss in people over 55 is age-related macular degeneration, or AMD, which afflicts an estimated 11 million Americans. As photoreceptors in the macula (the central part of the retina) die off, patients experience increasingly severe blurring, dimming, distortions, and blank spots in one or both eyes.
The disorder comes in two varieties, "wet" and "dry," both driven by a complex interaction of genetic, environmental, and lifestyle factors. It begins when deposits of cellular debris accumulate beneath the retinal pigment epithelium (RPE)—a layer of cells that nourish and remove waste products from the photoreceptors above them. In wet AMD, this process triggers the growth of abnormal, leaky blood vessels that damage the photoreceptors. In dry AMD, which accounts for 80 to 90 percent of cases, RPE cells atrophy, causing photoreceptors to wither away. Wet AMD can be controlled in about a quarter of patients, usually by injections of medication into the eye. For dry AMD, no effective remedy exists.
Stem Cells: Promise and Perils
Over the past decade, stem cell therapy has been widely touted as a potential treatment for AMD. The idea is to augment a patient's ailing RPE cells with healthy ones grown in the lab. A few small clinical trials have shown promising results. In a study published in 2018, for example, a University of Southern California team cultivated RPE tissue from embryonic stem cells on a plastic matrix and transplanted it into the retinas of four patients with advanced dry AMD. Because the trial was designed to test safety rather than efficacy, lead researcher Amir Kashani told a reporter, "we didn't expect that replacing RPE cells would return a significant amount of vision." Yet acuity improved substantially in one recipient, and the others regained their lost ability to focus on an object.
Therapies based on embryonic stem cells, however, have two serious drawbacks: Using fetal cell lines raises ethical issues, and such treatments require the patient to take immunosuppressant drugs (which can cause health problems of their own) to prevent rejection. That's why some experts favor a different approach—one based on induced pluripotent stem cells (iPSCs). Such cells, first produced in 2006, are made by returning adult cells to an undifferentiated state, and then using chemicals to reprogram them as desired. Treatments grown from a patient's own tissues could sidestep both hurdles associated with embryonic cells.
At least hypothetically. Today, the only stem cell therapies approved by the U.S. Food and Drug Administration (FDA) are umbilical cord-derived products for various blood and immune disorders. Although scientists are probing the use of embryonic stem cells or iPSCs for conditions ranging from diabetes to Parkinson's disease, such applications remain experimental—or fraudulent, as a growing number of patients treated at unlicensed "stem cell clinics" have painfully learned. (Some have gone blind after receiving bogus AMD therapies at those facilities.)
Last December, researchers at the National Eye Institute in Bethesda, Maryland, began enrolling patients with dry AMD in the country's first clinical trial using tissue grown from the patients' own stem cells. Led by biologist Kapil Bharti, the team intends to implant custom-made RPE cells in 12 recipients. If the effort pans out, it could someday save the sight of countless oldsters.
That, however, is what's technically referred to as a very big "if."
The First Steps
Bharti's trial is not the first in the world to use patient-derived iPSCs to treat age-related macular degeneration. In 2013, Japanese researchers implanted such cells into the eyes of a 77-year-old woman with wet AMD; after a year, her vision had stabilized, and she no longer needed injections to keep abnormal blood vessels from forming. A second patient was scheduled for surgery—but the procedure was canceled after the lab-grown RPE cells showed signs of worrisome mutations. That incident illustrates one potential problem with using stem cells: Under some circumstances, the cells or the tissue they form could turn cancerous.
"The knowledge and expertise we're gaining can be applied to many other iPSC-based therapies."
Bharti and his colleagues have gone to great lengths to avoid such outcomes. "Our process is significantly different," he told me in a phone interview. His team begins with patients' blood stem cells, which appear to be more genomically stable than the skin cells that the Japanese group used. After converting the blood cells to RPE stem cells, his team cultures them in a single layer on a biodegradable scaffold, which helps them grow in an orderly manner. "We think this material gives us a big advantage," Bharti says. The team uses a machine-learning algorithm to identify optimal cell structure and ensure quality control.
It takes about six months for a patch of iPSCs to become viable RPE cells. When they're ready, a surgeon uses a specially-designed tool to insert the tiny structure into the retina. Within days, the scaffold melts away, enabling the transplanted RPE cells to integrate fully into their new environment. Bharti's team initially tested their method on rats and pigs with eye damage mimicking AMD. The study, published in January 2019 in Science Translational Medicine, found that at ten weeks, the implanted RPE cells continued to function normally and protected neighboring photoreceptors from further deterioration. No trace of mutagenesis appeared.
Encouraged by these results, Bharti began recruiting human subjects. The Phase 1 trial will likely run through 2022, followed by a larger Phase 2 trial that could last another two or three years. FDA approval would require an even larger Phase 3 trial, with a decision expected sometime between 2025 and 2028—that is, if nothing untoward happens before then. One unknown (among many) is whether implanted cells can thrive indefinitely under the biochemically hostile conditions of an eye with AMD.
"Most people don't have a sense of just how long it takes to get something like this to work, and how many failures—even disasters—there are along the way," says Marco Zarbin, professor and chair of Ophthalmology and visual science at Rutgers New Jersey Medical School and co-editor of the book Cell-Based Therapy for Degenerative Retinal Diseases. "The first kidney transplant was done in 1933. But the first successful kidney transplant was in 1954. That gives you a sense of the time frame. We're really taking the very first steps in this direction."
Looking Ahead
Even if Bharti's method proves safe and effective, there's the question of its practicality. "My sense is that using induced pluripotent stem cells to treat the patient from whom they're derived is a very expensive undertaking," Zarbin observes. "So you'd have to have a very dramatic clinical benefit to justify that cost."
Bharti concedes that the price of iPSC therapy is likely to be high, given that each "dose" is formulated for a single individual, requires months to manufacture, and must be administered via microsurgery. Still, he expects economies of scale and production to emerge with time. "We're working on automating several steps of the process," he explains. "When that kicks in, a technician will be able to make products for 10 or 20 people at once, so the cost will drop proportionately."
Meanwhile, other researchers are pressing ahead with therapies for AMD using embryonic stem cells, which could be mass-produced to treat any patient who needs them. But should that approach eventually win FDA approval, Bharti believes there will still be room for a technique that requires neither fetal cell lines nor immunosuppression.
And not only for eye ailments. "The knowledge and expertise we're gaining can be applied to many other iPSC-based therapies," says the scientist, who is currently consulting with several companies that are developing such treatments. "I'm hopeful that we can leverage these approaches for a wide range of applications, whether it's for vision or across the body."
NEI launches iPS cell therapy trial for dry AMD
At the “Apple Store of Doctor’s Offices,” Preventive Care Is High Tech. Is it Worth $150 a Month?
A patient getting examined at health startup Forward.
What if going to the doctor's office could be … nice?
If you didn't have to wait for your appointment, but were ushered right in; if your medical data was all collated and easily searchable on an iPhone app; if a remote scribe took notes while you spoke with your doctor so you could make eye contact with them; if your doctor didn't seem horribly rushed.
Would you go to the doctor to get help staying healthy, rather than just to stop being sick?
Would that change the way you thought about your health? Would you go to the doctor to get help staying healthy, rather than just to stop being sick? And would that, in the long run, be much better for you?
Those are the animating questions for Forward, a healthcare startup devoted to preventive care. Led by founder Adrian Aoun, formerly of Google/Sidewalk labs, Forward opened its first office in San Francisco in 2016 and has since expanded to Los Angeles, Orange County, New York, and Washington, D.C., with a San Diego location opening soon.
It's been described as the "Apple Store of doctor's offices," which in some ways is a reaction to Forward's vibe: Patients have described the offices as having blonde wood, minimalist design, sparkling water on tap — and lots of high-tech gadgets, like the full-body scanner that replaces the standard scale and stethoscope.

The interior of a Forward office.
(Courtesy Forward)
The more crucial difference, though, is its model of care. Forward doesn't take insurance. Instead, patients, or "members," pay a flat $149 per month, along the lines of a subscription service like Netflix or a gym membership. That fee covers visits, messaging with medical staff through the Forward app, the use of a wearable (like a Fitbit or a sleep tracker) if the physician recommends it, plus any bloodwork or diagnostic tests run in the on-site labs. (The company declined to disclose how many people have signed up for memberships.)
Predictability is Forward's other significant, distinguishing feature: No surprise co-pays, or extra charges showing up on a billing statement months later. Everything is wrapped up in the $149 membership fee, unless the physician recommends visiting an outside specialist.
That caveat isn't a small one. It's important to note that Forward is in no way meant to replace standard health insurance. The service is strictly focused on preventive care, so it wouldn't be much use in case of an emergency; it's meant to help people, as far as is possible, avoid that emergency at all.
Ani Okkasian's family recently went through such an emergency. Her 62-year-old father, an active and seemingly healthy man living with diabetes, had been feeling unwell for a while, but struggled to receive constructive follow-up or tests from his doctor. It finally emerged that his liver was severely damaged, and he suffered a stroke — the risk of which can be elevated by liver disease. He seemed to deteriorate completely within mere weeks, Okkasian said, and in January he passed away.
"He was someone who'd go to the doctor regularly and listen to what they said and follow it," Okkasian said. "I shouldn't have had to bury my father at 62. I still believe to my core that his death could have been avoided if the primary care was adequate."
"I could tell that the people who designed [Forward] had lost someone to the legacy system; it was so streamlined and so much clearer."
Okkasian began researching, looking for a better alternative, and discovered Forward. Founder Aoun lost his grandfather to a heart attack; his brother's heart attack at age 31 was the impetus to start Forward.
"I could tell that that was the genesis," Okkasian said. "Having just lost someone, and having had to deal with different aspects of the healthcare industry — how complicated and convoluted that all is — I could tell that the people who designed [Forward] had lost someone to the legacy system; it was so streamlined and so much clearer."
So Who Is Forward For?
The Affordable Care Act mandates that evidence-based preventive care must be covered by insurers without any cost to the patient. Today, 30 million Americans are still living without health insurance; but for most of the population, cost shouldn't prevent access to standard, preventive care, says Benjamin Sommers, a physician and professor at the Harvard T.H. Chan School of Public Health who has studied the effect of the ACA on preventive care access.
For Okkasian and her family, it wasn't a lack of access to primary care that was at issue; it was the quality of that primary care. In 2019, that's probably true for a lot of people.
"How come all other industries have been disturbed except the medical industry?" Okkasian asked. "It's disturbing the most people. We're so advanced in so many ways, but when it comes to the healthcare system, we're not prioritizing the wellness of a person."
Is Forward the answer? Well, probably not for everyone. Its office are only in a handful of cities, and there are limits to how scalable it would be; it's unavoidable that the $149 per month charge restricts access for a lot of people. Those who have insurance through their employer might have a flexible spending account (FSA) that would cover some or all of the membership fee, and Forward has said that 15 percent of their early members came from underserved communities and were offered free plans; but for many others, that's just an unworkable extra cost.
Sommers also sounded a dubious note about a maximalist attitude toward data collection.
"Even though some patients may think that 'more is always better' — more testing, more screening, etc. — this isn't true," he said. "Some types of cancer screening, ovarian cancer screening for instance, are actually harmful or of no benefit, because studies have shown that they don't improve survival or health outcomes, but can lead to unnecessary testing, pain, false positives, anxiety, and other side effects.
"It's really great for people who are in good health, looking to make it better."
"I'm generally skeptical of efforts to charge people more to get 'extra testing' that isn't currently supported by the medical evidence," he added.
But relatively healthy people who want to take a more active approach to their health — or people who have frequent testing needs, like those using the HIV-prevention drug PrEP, and want to avoid co-pays — might benefit from the on-demand, low-friction experience that Forward offers.
"It's really great for people who are in good health, looking to make it better," Okkasian said. "Your experience is simplified to a point where you feel empowered, not scared."
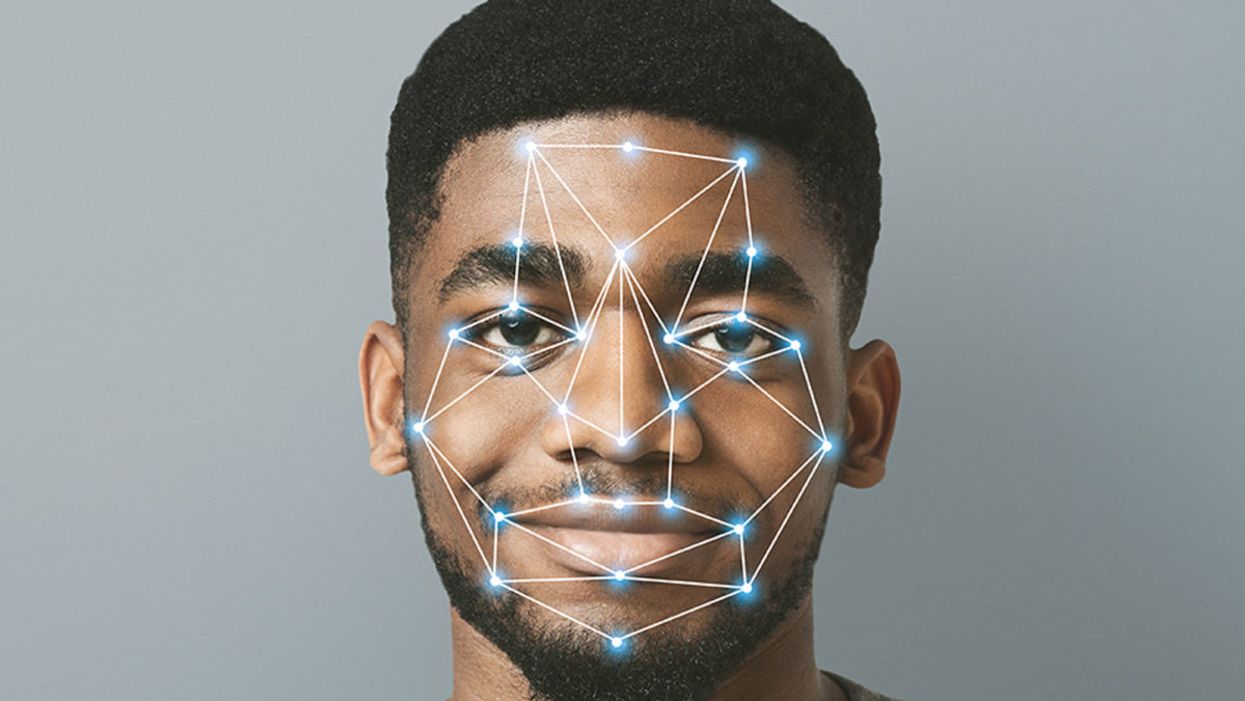
Facial Recognition Can Reduce Racial Profiling and False Arrests
The use of face recognition technology is expanding exponentially right now.
[Editor's Note: This essay is in response to our current Big Question, which we posed to experts with different perspectives: "Do you think the use of facial recognition technology by the police or government should be banned? If so, why? If not, what limits, if any, should be placed on its use?"]
Opposing facial recognition technology has become an article of faith for civil libertarians. Many who supported the bans in cities like San Francisco and Oakland have declared the technology to be inherently racist and abusive.
The greatest danger would be to categorically oppose this technology and pretend that it will simply go away.
I have spent my career as a criminal defense attorney and a civil libertarian -- and I do not fear it. Indeed, I see it as positive so long as it is appropriately regulated and controlled.
We are living in the beginning of a biometric age, where technology uses our physical or biological characteristics for a variety of products and services. It holds great promises as well as great risks. The greatest danger, however, would be to categorically oppose this technology and pretend that it will simply go away.
This is an age driven as much by consumer as it is government demand. Living in denial may be emotionally appealing, but it will only hasten the creation of post-privacy world. If we do not address this emerging technology, movements in public will increasingly result in instant recognition and even tracking. It is the type of fish-bowl society that strips away any expectation of privacy in our interactions and associations.
The biometrics field is expanding exponentially, largely due to the popularity of consumer products using facial recognition technology (FRT) -- from the iPhone program to shopping ones that recognize customers.
But the privacy community is losing this battle because it is using the privacy rationales and doctrines forged in the earlier electronic surveillance periods. Just as generals are often accused of planning to fight the last war, civil libertarians can sometimes cling to past models despite their decreasing relevance in the current world.
I see FRT as having positive implications that are worth pursuing. When properly used, biometrics can actually enhance privacy interests and even reduce racial profiling by reducing false arrests and the warrantless "patdowns" allowed by the Supreme Court. Bans not only deny police a technology widely used by businesses, but return police to the highly flawed default of "eye balling" suspects -- a system with a considerably higher error rate than top FRT programs.
Officers are often wrong and stop a great number of suspects in the hopes of finding a wanted felon.
A study in Australia showed that passport officers who had taken photographs of subjects in ideal conditions nonetheless experienced high error rates when identifying them shortly afterward, including 14 percent false acceptance rates. Currently, officers stop suspects based on their memory from seeing a photograph days or weeks earlier. They are often wrong and stop a great number of suspects in the hopes of finding a wanted felon. The best FRT programs achieve an astonishing accuracy rate, though real-world implementation has challenges that must be addressed.
One legitimate concern raised in early studies showed higher error rates in recognitions for certain groups, particularly African American women. An MIT study finding that error rate prompted major improvements in the algorithms as well as training changes to greatly reduce the frequency of errors. The issue remains a concern, but there is nothing inherently racist in algorithms. These are a set of computer instructions that isolate and process with the parameters and conditions set by creators.
To be sure, there is room for improvement in some algorithms. Tests performed by the American Civil Liberties Union (ACLU) reportedly showed only an 80 percent accuracy rate in comparing mug shots to pictures of members of Congress when using Amazon's "Rekognition" system. It recently showed the same 80 percent rate in doing the same comparison to members of the California legislators.
However, different algorithms are available with differing levels of performance. Moreover, these products can be set with a lower discrimination level. The fact is that the top algorithms tested by the National Institute of Standards and Technology showed that their accuracy rate is greater than 99 percent.
The greatest threat of biometric technologies is to democratic values.
Assuming a top-performing algorithm is used, the result could be highly beneficial for civil liberties as opposed to the alternative of "eye balling" suspects. Consider the Boston Bombing where police declared a "containment zone" and forced families into the street with their hands in the air.
The suspect, Dzhokhar Tsarnaev, moved around Boston and was ultimately found outside the "containment zone" once authorities abandoned near martial law. He was caught on some surveillance systems but not identified. FRT can help law enforcement avoid time-consuming area searches and the questionable practice of forcing people out of their homes to physically examine them.
If we are to avoid a post-privacy world, we will have to redefine what we are trying to protect and reconceive how we hope to protect it. In my view, the greatest threat of biometric technologies is to democratic values. Authoritarian nations like China have made huge investments into FRT precisely because they know that the threat of recognition in public deters citizens from associating or interacting with protesters or dissidents. Recognition changes conduct. That chilling effect is what we have the worry about the most.
Conventional privacy doctrines do not offer much protection. The very concept of "public privacy" is treated as something of an oxymoron by courts. Public acts and associations are treated as lacking any reasonable expectation of privacy. In the same vein, the right to anonymity is not a strong avenue for protection. We are not living in an anonymous world anymore.
Consumers want products like FaceFind, which link their images with others across social media. They like "frictionless" transactions and authentications using faceprints. Despite the hyperbole in places like San Francisco, civil libertarians will not succeed in getting that cat to walk backwards.
The basis for biometric privacy protection should not be focused on anonymity, but rather obscurity. You will be increasingly subject to transparency-forcing technology, but we can legislatively mandate ways of obscuring that information. That is the objective of the Biometric Privacy Act that I have proposed in recent research. However, no such comprehensive legislation has passed through Congress.
The ability to spot fraudulent entries at airports or recognizing a felon in flight has obvious benefits for all citizens.
We also need to recognize that FRT has many beneficial uses. Biometric guns can reduce accidents and criminals' conduct. New authentications using FRT and other biometric programs could reduce identity theft.
And, yes, FRT could help protect against unnecessary police stops or false arrests. Finally, and not insignificantly, this technology could stop serious crimes, from terrorist attacks to the capturing of dangerous felons. The ability to spot fraudulent entries at airports or recognizing a felon in flight has obvious benefits for all citizens.
We can live and thrive in a biometric era. However, we will need to bring together civil libertarians with business and government experts if we are going to control this technology rather than have it control us.
[Editor's Note: Read the opposite perspective here.]