Researchers Behaving Badly: Known Frauds Are "the Tip of the Iceberg"

Disgraced stem cell researcher and celebrity surgeon Paolo Macchiarini.
Last week, the whistleblowers in the Paolo Macchiarini affair at Sweden's Karolinska Institutet went on the record here to detail the retaliation they suffered for trying to expose a star surgeon's appalling research misconduct.
Scientific fraud of the type committed by Macchiarini is rare, but studies suggest that it's on the rise.
The whistleblowers had discovered that in six published papers, Macchiarini falsified data, lied about the condition of patients and circumvented ethical approvals. As a result, multiple patients suffered and died. But Karolinska turned a blind eye for years.
Scientific fraud of the type committed by Macchiarini is rare, but studies suggest that it's on the rise. Just this week, for example, Retraction Watch and STAT together broke the news that a Harvard Medical School cardiologist and stem cell researcher, Piero Anversa, falsified data in a whopping 31 papers, which now have to be retracted. Anversa had claimed that he could regenerate heart muscle by injecting bone marrow cells into damaged hearts, a result that no one has been able to duplicate.
A 2009 study published in the Public Library of Science (PLOS) found that about two percent of scientists admitted to committing fabrication, falsification or plagiarism in their work. That's a small number, but up to one third of scientists admit to committing "questionable research practices" that fall into a gray area between rigorous accuracy and outright fraud.
These dubious practices may include misrepresentations, research bias, and inaccurate interpretations of data. One common questionable research practice entails formulating a hypothesis after the research is done in order to claim a successful premise. Another highly questionable practice that can shape research is ghost-authoring by representatives of the pharmaceutical industry and other for-profit fields. Still another is gifting co-authorship to unqualified but powerful individuals who can advance one's career. Such practices can unfairly bolster a scientist's reputation and increase the likelihood of getting the work published.
The above percentages represent what scientists admit to doing themselves; when they evaluate the practices of their colleagues, the numbers jump dramatically. In a 2012 study published in the Journal of Research in Medical Sciences, researchers estimated that 14 percent of other scientists commit serious misconduct, while up to 72 percent engage in questionable practices. While these are only estimates, the problem is clearly not one of just a few bad apples.
In the PLOS study, Daniele Fanelli says that increasing evidence suggests the known frauds are "just the 'tip of the iceberg,' and that many cases are never discovered" because fraud is extremely hard to detect.
Essentially everyone wants to be associated with big breakthroughs, and they may overlook scientifically shaky foundations when a major advance is claimed.
In addition, it's likely that most cases of scientific misconduct go unreported because of the high price of whistleblowing. Those in the Macchiarini case showed extraordinary persistence in their multi-year campaign to stop his deadly trachea implants, while suffering serious damage to their careers. Such heroic efforts to unmask fraud are probably rare.
To make matters worse, there are numerous players in the scientific world who may be complicit in either committing misconduct or covering it up. These include not only primary researchers but co-authors, institutional executives, journal editors, and industry leaders. Essentially everyone wants to be associated with big breakthroughs, and they may overlook scientifically shaky foundations when a major advance is claimed.
Another part of the problem is that it's rare for students in science and medicine to receive an education in ethics. And studies have shown that older, more experienced and possibly jaded researchers are more likely to fudge results than their younger, more idealistic colleagues.
So, given the steep price that individuals and institutions pay for scientific misconduct, what compels them to go down that road in the first place? According to the JRMS study, individuals face intense pressures to publish and to attract grant money in order to secure teaching positions at universities. Once they have acquired positions, the pressure is on to keep the grants and publishing credits coming in order to obtain tenure, be appointed to positions on boards, and recruit flocks of graduate students to assist in research. And not to be underestimated is the human ego.
Paolo Macchiarini is an especially vivid example of a scientist seeking not only fortune, but fame. He liberally (and falsely) claimed powerful politicians and celebrities, even the Pope, as patients or admirers. He may be an extreme example, but we live in an age of celebrity scientists who bring huge amounts of grant money and high prestige to the institutions that employ them.
The media plays a significant role in both glorifying stars and unmasking frauds. In the Macchiarini scandal, the media first lifted him up, as in NBC's laudatory documentary, "A Leap of Faith," which painted him as a kind of miracle-worker, and then brought him down, as in the January 2016 documentary, "The Experiments," which chronicled the agonizing death of one of his patients.
Institutions can also play a crucial role in scientific fraud by putting more emphasis on the number and frequency of papers published than on their quality. The whole course of a scientist's career is profoundly affected by something called the h-index. This is a number based on both the frequency of papers published and how many times the papers are cited by other researchers. Raising one's ranking on the h-index becomes an overriding goal, sometimes eclipsing the kind of patient, time-consuming research that leads to true breakthroughs based on reliable results.
Universities also create a high-pressured environment that encourages scientists to cut corners. They, too, place a heavy emphasis on attracting large monetary grants and accruing fame and prestige. This can lead them, just as it led Karolinska, to protect a star scientist's sloppy or questionable research. According to Dr. Andrew Rosenberg, who is director of the Center for Science and Democracy at the U.S.-based Union of Concerned Scientists, "Karolinska defended its investment in an individual as opposed to the long-term health of the institution. People were dying, and they should have outsourced the investigation from the very beginning."
Having institutions investigate their own practices is a conflict of interest from the get-go, says Rosenberg.
Scientists, universities, and research institutions are also not immune to fads. "Hot" subjects attract grant money and confer prestige, incentivizing scientists to shift their research priorities in a direction that garners more grants. This can mean neglecting the scientist's true area of expertise and interests in favor of a subject that's more likely to attract grant money. In Macchiarini's case, he was allegedly at the forefront of the currently sexy field of regenerative medicine -- a field in which Karolinska was making a huge investment.
The relative scarcity of resources intensifies the already significant pressure on scientists. They may want to publish results rapidly, since they face many competitors for limited grant money, academic positions, students, and influence. The scarcity means that a great many researchers will fail while only a few succeed. Once again, the temptation may be to rush research and to show it in the most positive light possible, even if it means fudging or exaggerating results.
Though the pressures facing scientists are very real, the problem of misconduct is not inevitable.
Intense competition can have a perverse effect on researchers, according to a 2007 study in the journal Science of Engineering and Ethics. Not only does it place undue pressure on scientists to succeed, it frequently leads to the withholding of information from colleagues, which undermines a system in which new discoveries build on the previous work of others. Researchers may feel compelled to withhold their results because of the pressure to be the first to publish. The study's authors propose that more investment in basic research from governments could alleviate some of these competitive pressures.
Scientific journals, although they play a part in publishing flawed science, can't be expected to investigate cases of suspected fraud, says the German science blogger Leonid Schneider. Schneider's writings helped to expose the Macchiarini affair.
"They just basically wait for someone to retract problematic papers," he says.
He also notes that, while American scientists can go to the Office of Research Integrity to report misconduct, whistleblowers in Europe have no external authority to whom they can appeal to investigate cases of fraud.
"They have to go to their employer, who has a vested interest in covering up cases of misconduct," he says.
Science is increasingly international. Major studies can include collaborators from several different countries, and he suggests there should be an international body accessible to all researchers that will investigate suspected fraud.
Ultimately, says Rosenberg, the scientific system must incorporate trust. "You trust co-authors when you write a paper, and peer reviewers at journals trust that scientists at research institutions like Karolinska are acting with integrity."
Without trust, the whole system falls apart. It's the trust of the public, an elusive asset once it has been betrayed, that science depends upon for its very existence. Scientific research is overwhelmingly financed by tax dollars, and the need for the goodwill of the public is more than an abstraction.
The Macchiarini affair raises a profound question of trust and responsibility: Should multiple co-authors be held responsible for a lead author's misconduct?
Karolinska apparently believes so. When the institution at last owned up to the scandal, it vindictively found Karl Henrik-Grinnemo, one of the whistleblowers, guilty of scientific misconduct as well. It also designated two other whistleblowers as "blameworthy" for their roles as co-authors of the papers on which Macchiarini was the lead author.
As a result, the whistleblowers' reputations and employment prospects have become collateral damage. Accusations of research misconduct can be a career killer. Research grants dry up, employment opportunities evaporate, publishing becomes next to impossible, and collaborators vanish into thin air.
Grinnemo contends that co-authors should only be responsible for their discrete contributions, not for the data supplied by others.
"Different aspects of a paper are highly specialized," he says, "and that's why you have multiple authors. You cannot go through every single bit of data because you don't understand all the parts of the article."
This is especially true in multidisciplinary, translational research, where there are sometimes 20 or more authors. "You have to trust co-authors, and if you find something wrong you have to notify all co-authors. But you couldn't go through everything or it would take years to publish an article," says Grinnemo.
Though the pressures facing scientists are very real, the problem of misconduct is not inevitable. Along with increased support from governments and industry, a change in academic culture that emphasizes quality over quantity of published studies could help encourage meritorious research.
But beyond that, trust will always play a role when numerous specialists unite to achieve a common goal: the accumulation of knowledge that will promote human health, wealth, and well-being.
[Correction: An earlier version of this story mistakenly credited The New York Times with breaking the news of the Anversa retractions, rather than Retraction Watch and STAT, which jointly published the exclusive on October 14th. The piece in the Times ran on October 15th. We regret the error.]
Want to Motivate Vaccinations? Message Optimism, Not Doom
There is a lot to be optimistic about regarding the new safe and highly effective vaccines, which are moving society closer toward the goal of close human contact once again.
After COVID-19 was declared a worldwide pandemic by the World Health Organization on March 11, 2020, life as we knew it altered dramatically and millions went into lockdown. Since then, most of the world has had to contend with masks, distancing, ventilation and cycles of lockdowns as surges flare up. Deaths from COVID-19 infection, along with economic and mental health effects from the shutdowns, have been devastating. The need for an ultimate solution -- safe and effective vaccines -- has been paramount.
On November 9, 2020 (just 8 months after the pandemic announcement), the press release for the first effective COVID-19 vaccine from Pfizer/BioNTech was issued, followed by positive announcements regarding the safety and efficacy of five other vaccines from Moderna, University of Oxford/AztraZeneca, Novavax, Johnson and Johnson and Sputnik V. The Moderna and Pfizer vaccines have earned emergency use authorization through the FDA in the United States and are being distributed. We -- after many long months -- are seeing control of the devastating COVID-19 pandemic glimmering into sight.
To be clear, these vaccine candidates for COVID-19, both authorized and not yet authorized, are highly effective and safe. In fact, across all trials and sites, all six vaccines were 100% effective in preventing hospitalizations and death from COVID-19.
All Vaccines' Phase 3 Clinical Data
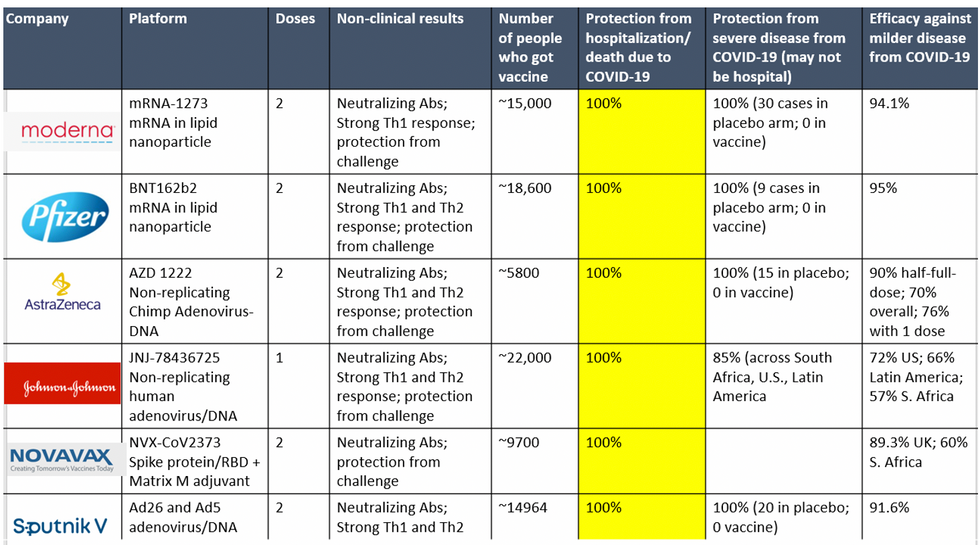
Complete protection against hospitalization and death from COVID-19 exhibited by all vaccines with phase 3 clinical trial data.
This astounding level of protection from SARS-CoV-2 from all vaccine candidates across multiple regions is likely due to robust T cell response from vaccination and will "defang" the virus from the concerns that led to COVID-19 restrictions initially: the ability of the virus to cause severe illness. This is a time of hope and optimism. After the devastating third surge of COVID-19 infections and deaths over the winter, we finally have an opportunity to stem the crisis – if only people readily accept the vaccines.
Amidst these incredible scientific advancements, however, public health officials and politicians have been pushing downright discouraging messaging. The ubiquitous talk of ongoing masks and distancing restrictions without any clear end in sight threatens to dampen uptake of the vaccines. It's imperative that we break down each concern and see if we can revitalize our public health messaging accordingly.
The first concern: we currently do not know if the vaccines block asymptomatic infection as well as symptomatic disease, since none of the phase 3 vaccine trials were set up to answer this question. However, there is biological plausibility that the antibodies and T-cell responses blocking symptomatic disease will also block asymptomatic infection in the nasal passages. IgG immunoglobulins (generated and measured by the vaccine trials) enter the nasal mucosa and systemic vaccinations generate IgA antibodies at mucosal surfaces. Monoclonal antibodies given to outpatients with COVID-19 hasten viral clearance from the airways.
Although it is prudent for those who are vaccinated to wear masks around the unvaccinated in case a slight risk of transmission remains, two fully vaccinated people can comfortably abandon masking around each other.
Moreover, data from the AztraZeneca trial (including in the phase 3 trial final results manuscript), where weekly self-swabbing was done by participants, and data from the Moderna trial, where a nasal swab was performed prior to the second dose, both showed risk reductions in asymptomatic infection with even a single dose. Finally, real-world data from a large Pfizer-based vaccine campaign in Israel shows a 50% reduction in infections (asymptomatic or symptomatic) after just the first dose.
Therefore, the likelihood of these vaccines blocking asymptomatic carriage, as well as symptomatic disease, is high. Although it is prudent for those who are vaccinated to wear masks around the unvaccinated in case a slight risk of transmission remains, two fully vaccinated people can comfortably abandon masking around each other. Moreover, as the percentage of vaccinated people increases, it will be increasingly untenable to impose restrictions on this group. Once herd immunity is reached, these restrictions can and should be abandoned altogether.
The second concern translating to "doom and gloom" messaging lately is around the identification of troubling new variants due to enhanced surveillance via viral sequencing. Four major variants circulating at this point (with others described in the past) are the B.1.1.7 variant ("UK variant"), B.1.351 ("South Africa variant), P.1. ("Brazil variant"), and the L452R variant identified in California. Although the UK variant is likely to be more transmissible, as is the South Africa variant, we have no reason to believe that masks, distancing and ventilation are ineffective against these variants.
Moreover, neutralizing antibody titers with the Pfizer and Moderna vaccines do not seem to be significantly reduced against the variants. Finally, although the Novavax 2-dose and Johnson and Johnson (J&J) 1-dose vaccines had lower rates of efficacy against moderate COVID-19 disease in South Africa, their efficacy against severe disease was impressively high. In fact J&J's vaccine still prevented 100% of hospitalizations and death from COVID-19. When combining both hospitalizations/deaths and severe symptoms managed at home, the J&J 1-dose vaccine was 85% protective across all three sites of the trial: the U.S., Latin America (including Brazil), and South Africa.
In South Africa, nearly all cases of COVID-19 (95%) were due to infection with the B.1.351 SARS-CoV-2 variant. Finally, since herd immunity does not rely on maximal immune responses among all individuals in a society, the Moderna/Pfizer/J&J vaccines are all likely to achieve that goal against variants. And thankfully, all of these vaccines can be easily modified to boost specifically against a new variant if needed (indeed, Moderna and Pfizer are already working on boosters against the prominent variants).
The third concern of some public health officials is that people will abandon all restrictions once vaccinated unless overly cautious messages are drilled into them. Indeed, the false idea that if you "give people an inch, they will take a mile" has been misinforming our messaging about mitigation since the beginning of the pandemic. For example, the very phrase "stay at home" with all of its non-applicability for essential workers and single individuals is stigmatizing and unrealistic for many. Instead, the message should have focused on how people can additively reduce their risks under different circumstances.
The public will be more inclined to trust health officials if those officials communicate with nuanced messages backed up by evidence, rather than with broad brushstrokes that shame. Therefore, we should be saying that "vaccinated people can be together with other vaccinated individuals without restrictions but must protect the unvaccinated with masks and distancing." And we can say "unvaccinated individuals should adhere to all current restrictions until vaccinated" without fear of misunderstandings. Indeed, this kind of layered advice has been communicated to people living with HIV and those without HIV for a long time (if you have HIV but partner does not, take these precautions; if both have HIV, you can do this, etc.).
Our heady progress in vaccine development, along with the incredible efficacy results of all of them, is unprecedented. However, we are at risk of undermining such progress if people balk at the vaccine because they don't believe it will make enough of a difference. One of the most critical messages we can deliver right now is that these vaccines will eventually free us from the restrictions of this pandemic. Let's use tiered messaging and clear communication to boost vaccine optimism and uptake, and get us to the goal of close human contact once again.
Inside Scoop: How a DARPA Scientist Helped Usher in a Game-Changing Covid Treatment
Amy Jenkins is a program manager for the Defense Advanced Research Projects Agency's Biological Technologies Office, which runs a project called the Pandemic Prevention Platform.
Amy Jenkins was in her office at DARPA, a research and development agency within the Department of Defense, when she first heard about a respiratory illness plaguing the Chinese city of Wuhan. Because she's a program manager for DARPA's Biological Technologies Office, her colleagues started stopping by. "It's really unusual, isn't it?" they would say.
At the time, China had a few dozen cases of what we now call COVID-19. "We should maybe keep an eye on that," she thought.
Early in 2020, still just keeping watch, she was visiting researchers working on DARPA's Pandemic Prevention Platform (P3), a project to develop treatments for "any known or previously unknown infectious threat," within 60 days of its appearance. "We looked at each other and said, 'Should we be doing something?'" she says.
For projects like P3, groups of scientists—often at universities and private companies—compete for DARPA contracts, and program managers like Jenkins oversee the work. Those that won the P3 bid included scientists at AbCellera Biologics, Inc., AstraZeneca, Duke University, and Vanderbilt University.
At the time Jenkins was talking to the P3 performers, though, they didn't have evidence of community transmission. "We would have to cross that bar before we considered doing anything," she says.
The world soon leapt far over that bar. By the time Jenkins and her team decided P3 should be doing something—with their real work beginning in late February--it was too late to prevent this pandemic. But she could help P3 dig into the chemical foundations of COVID-19's malfeasance, and cut off its roots. That work represents, in fact, her roots.
In late February 2020, DARPA received a single blood sample from a recovered COVID-19 patient, in which P3 researchers could go fishing for antibodies. The day it arrived, Jenkins's stomach roiled. "We get one shot," she thought.
Fighting the Smallest Enemies
Jenkins, who's in her early 40s, first got into germs the way many 90s kids did: by reading The Hot Zone, a novel about a hemorrhagic fever gone rogue. It wasn't exactly the disintegrating organs that hooked her. It was the idea that "these very pathogens that we can't even see can make us so sick and bring us to our knees," she says. Reading about scientists facing down deadly disease, she wondered, "How do these things make you so sick?"
She chased that question in college, majoring in both biomolecular science and chemistry, and later became an antibody expert. Antibodies are proteins that hook to a pathogen to block it from attaching to your cells, or tag it for destruction by the rest of the immune system. Soon, she jumped on the "monoclonal antibodies" train—developing synthetic versions of these natural defenses, which doctors can give to people to help them battle an early-stage infection, and even to prevent an infection from taking root after an exposure.
Jenkins likens the antibody treatments to the old aphorism about fishing: Vaccines teach your body how to fish, but antibodies simply give your body the pesca-fare. While that, as the saying goes, won't feed you for a lifetime, it will last a few weeks or months. Monoclonal antibodies thus are a promising preventative option in the immediate short-term when a vaccine hasn't yet been given (or hasn't had time to produce an immune response), as well as an important treatment weapon in the current fight. After former president Donald Trump contracted COVID-19, he received a monoclonal antibody treatment from biotech company Regeneron.
As for Jenkins, she started working as a DARPA Biological Technologies Office contractor soon after completing her postdoc. But it was a suit job, not a labcoat job. And suit jobs, at first, left Jenkins conflicted, worried about being bored. She'd give it a year, she thought. But the year expired, and bored she was not. Around five years later, in June 2019, the agency hired her to manage several of the office's programs. A year into that gig, the world was months into a pandemic.
The Pandemic Pivot
At DARPA, Jenkins inherited five programs, including P3. P3 works by taking blood from recovered people, fishing out their antibodies, identifying the most effective ones, and then figuring out how to manufacture them fast. Back then, P3 existed to help with nebulous, future outbreaks: Pandemic X. Not this pandemic. "I did not have a crystal ball," she says, "but I will say that all of us in the infectious diseases and public-health realm knew that the next pandemic was coming."
Three days after a January 2020 meeting with P3 researchers, COVID-19 appeared in Seattle, then began whipping through communities. The time had come for P3 teams to swivel. "We had done this," she says. "We had practiced this before." But would their methods stand up to something unknown, racing through the global population? "The big anxiety was, 'Wow, this was real,'" says Jenkins.
While facing down that realness, Jenkins was also managing other projects. In one called PREPARE, groups develop "medical countermeasures" that modulate a person's genetic code to boost their bodies' responses to threats. Another project, NOW, envisions shipping-container-sized factories that can make thousands of vaccine doses in days. And then there's Prometheus—which means "forethought" in Greek, and is the name of the god who stole fire and gave it to humans. Wrapping up as COVID ramped up, Prometheus aimed to identify people who are contagious—with whatever—before they start coughing, and even if they never do.
All of DARPA's projects focus on developing early-stage technology, passing it off to other agencies or industry to put it into operation. The orientation toward a specific goal appealed to Jenkins, as a contrast to academia. "You go down a rabbit hole for years at a time sometimes, chasing some concept you found interesting in the lab," she says. That's good for the human pursuit of knowledge, and leads to later applications, but DARPA wants a practical prototype—stat.
"Dual-Use" Technologies
That desire, though, and the fact that DARPA is a defense agency, present philosophical complications. "Bioethics in the national-security context turns all the dials up to 10+," says Jonathan Moreno, a medical ethicist at the University of Pennsylvania.
While developing antibody treatments to stem a pandemic seems straightforwardly good, all biological research—especially that backed by military money—requires evaluating potential knock-on applications, even those that might come from outside the entity that did the developing. As Moreno put it, "Albert Einstein wasn't thinking about blowing up Hiroshima." Particularly sensitive are so-called "dual-use" technologies—those tools that could be used for both benign and nefarious purposes, or are of interest to both the civilian and military worlds.
Moreno takes Prometheus itself as an example of "dual-use" technology. "Think about somebody wearing a suicide vest. Instead of a suicide vest, make them extremely contagious with something. The flu plus Ebola," he says. "Send them someplace, a sensitive environment. We would like to be able to defend against that"—not just tell whether Uncle Fred is bringing asymptomatic COVID home for Christmas. Prometheus, Jenkins says, had safety in mind from the get-go, and required contenders to "develop a risk mitigation plan" and "detail their strategy for appropriate control of information."
To look at a different program, if you can modulate genes to help healing, you probably know something (or know someone else could infer something) about how to hinder healing. Those sorts of risks are why PREPARE researchers got their own "ethical, legal, and social implications" panel, which meets quarterly "to ensure that we are performing all research and publications in a safe and ethical manner," says Jenkins.
DARPA as a whole, Moreno says, is institutionally sensitive to bioethics. The agency has ethics panels, and funded a 2014 National Academies assessment of how to address the "ethical, legal, and societal issues" around technology that has military relevance. "In the cases of biotechnologies where some of that research brushes up against what could legitimately be considered dual-use, that in itself justifies our investment," says Jenkins. "DARPA deliberately focuses on safety and countermeasures against potentially dangerous technologies, and we structure our programs to be transparent, safe, and legal."
Going Fishing
In late February 2020, DARPA received a single blood sample from a recovered COVID-19 patient, in which P3 researchers could go fishing for antibodies. The day it arrived, Jenkins's stomach roiled. "We get one shot," she thought.
As scientists from the P3-funded AbCellera went through the processes they'd practiced, Jenkins managed their work, tracking progress and relaying results. Soon, the team had isolated a suitable protein: bamlanivimab. It attaches to and blocks off the infamous spike proteins on SARS-CoV-2—those sticky suction-cups in illustrations. Partnering with Eli Lilly in a manufacturing agreement, the biotech company brought it to clinical trials in May, just a few months after its work on the deadly pathogen began, after much of the planet became a hot zone.
On November 10—Jenkins's favorite day at the (home) office—the FDA provided Eli Lilly emergency use authorization for bamlanivimab. But she's only mutedly screaming (with joy) inside her heart. "This pandemic isn't 'one morning we're going to wake up and it's all over,'" she says. When it is over, she and her colleagues plan to celebrate their promethean work. "I'm hoping to be able to do it in person," she says. "Until then, I have not taken a breath."

