Scientists Are Building an “AccuWeather” for Germs to Predict Your Risk of Getting the Flu

A future app may help you avoid getting the flu by informing you of your local risk on a given day.
Applied mathematician Sara del Valle works at the U.S.'s foremost nuclear weapons lab: Los Alamos. Once colloquially called Atomic City, it's a hidden place 45 minutes into the mountains northwest of Santa Fe. Here, engineers developed the first atomic bomb.
Like AccuWeather, an app for disease prediction could help people alter their behavior to live better lives.
Today, Los Alamos still a small science town, though no longer a secret, nor in the business of building new bombs. Instead, it's tasked with, among other things, keeping the stockpile of nuclear weapons safe and stable: not exploding when they're not supposed to (yes, please) and exploding if someone presses that red button (please, no).
Del Valle, though, doesn't work on any of that. Los Alamos is also interested in other kinds of booms—like the explosion of a contagious disease that could take down a city. Predicting (and, ideally, preventing) such epidemics is del Valle's passion. She hopes to develop an app that's like AccuWeather for germs: It would tell you your chance of getting the flu, or dengue or Zika, in your city on a given day. And like AccuWeather, it could help people alter their behavior to live better lives, whether that means staying home on a snowy morning or washing their hands on a sickness-heavy commute.

Sara del Valle of Los Alamos is working to predict and prevent epidemics using data and machine learning.
Since the beginning of del Valle's career, she's been driven by one thing: using data and predictions to help people behave practically around pathogens. As a kid, she'd always been good at math, but when she found out she could use it to capture the tentacular spread of disease, and not just manipulate abstractions, she was hooked.
When she made her way to Los Alamos, she started looking at what people were doing during outbreaks. Using social media like Twitter, Google search data, and Wikipedia, the team started to sift for trends. Were people talking about hygiene, like hand-washing? Or about being sick? Were they Googling information about mosquitoes? Searching Wikipedia for symptoms? And how did those things correlate with the spread of disease?
It was a new, faster way to think about how pathogens propagate in the real world. Usually, there's a 10- to 14-day lag in the U.S. between when doctors tap numbers into spreadsheets and when that information becomes public. By then, the world has moved on, and so has the disease—to other villages, other victims.
"We found there was a correlation between actual flu incidents in a community and the number of searches online and the number of tweets online," says del Valle. That was when she first let herself dream about a real-time forecast, not a 10-days-later backcast. Del Valle's group—computer scientists, mathematicians, statisticians, economists, public health professionals, epidemiologists, satellite analysis experts—has continued to work on the problem ever since their first Twitter parsing, in 2011.
They've had their share of outbreaks to track. Looking back at the 2009 swine flu pandemic, they saw people buying face masks and paying attention to the cleanliness of their hands. "People were talking about whether or not they needed to cancel their vacation," she says, and also whether pork products—which have nothing to do with swine flu—were safe to buy.
At the latest meeting with all the prediction groups, del Valle's flu models took first and second place.
They watched internet conversations during the measles outbreak in California. "There's a lot of online discussion about anti-vax sentiment, and people trying to convince people to vaccinate children and vice versa," she says.
Today, they work on predicting the spread of Zika, Chikungunya, and dengue fever, as well as the plain old flu. And according to the CDC, that latter effort is going well.
Since 2015, the CDC has run the Epidemic Prediction Initiative, a competition in which teams like de Valle's submit weekly predictions of how raging the flu will be in particular locations, along with other ailments occasionally. Michael Johannson is co-founder and leader of the program, which began with the Dengue Forecasting Project. Its goal, he says, was to predict when dengue cases would blow up, when previously an area just had a low-level baseline of sick people. "You'll get this massive epidemic where all of a sudden, instead of 3,000 to 4,000 cases, you have 20,000 cases," he says. "They kind of come out of nowhere."
But the "kind of" is key: The outbreaks surely come out of somewhere and, if scientists applied research and data the right way, they could forecast the upswing and perhaps dodge a bomb before it hit big-time. Questions about how big, when, and where are also key to the flu.
A big part of these projects is the CDC giving the right researchers access to the right information, and the structure to both forecast useful public-health outcomes and to compare how well the models are doing. The extra information has been great for the Los Alamos effort. "We don't have to call departments and beg for data," says del Valle.
When data isn't available, "proxies"—things like symptom searches, tweets about empty offices, satellite images showing a green, wet, mosquito-friendly landscape—are helpful: You don't have to rely on anyone's health department.
At the latest meeting with all the prediction groups, del Valle's flu models took first and second place. But del Valle wants more than weekly numbers on a government website; she wants that weather-app-inspired fortune-teller, incorporating the many diseases you could get today, standing right where you are. "That's our dream," she says.

This plot shows the the correlations between the online data stream, from Wikipedia, and various infectious diseases in different countries. The results of del Valle's predictive models are shown in brown, while the actual number of cases or illness rates are shown in blue.
(Courtesy del Valle)
The goal isn't to turn you into a germophobic agoraphobe. It's to make you more aware when you do go out. "If you know it's going to rain today, you're more likely to bring an umbrella," del Valle says. "When you go on vacation, you always look at the weather and make sure you bring the appropriate clothing. If you do the same thing for diseases, you think, 'There's Zika spreading in Sao Paulo, so maybe I should bring even more mosquito repellent and bring more long sleeves and pants.'"
They're not there yet (don't hold your breath, but do stop touching your mouth). She estimates it's at least a decade away, but advances in machine learning could accelerate that hypothetical timeline. "We're doing baby steps," says del Valle, starting with the flu in the U.S., dengue in Brazil, and other efforts in Colombia, Ecuador, and Canada. "Going from there to forecasting all diseases around the globe is a long way," she says.
But even AccuWeather started small: One man began predicting weather for a utility company, then helping ski resorts optimize their snowmaking. His influence snowballed, and now private forecasting apps, including AccuWeather's, populate phones across the planet. The company's progression hasn't been without controversy—privacy incursions, inaccuracy of long-term forecasts, fights with the government—but it has continued, for better and for worse.
Disease apps, perhaps spun out of a small, unlikely team at a nuclear-weapons lab, could grow and breed in a similar way. And both the controversies and public-health benefits that may someday spin out of them lie in the future, impossible to predict with certainty.
Is a Successful HIV Vaccine Finally on the Horizon?
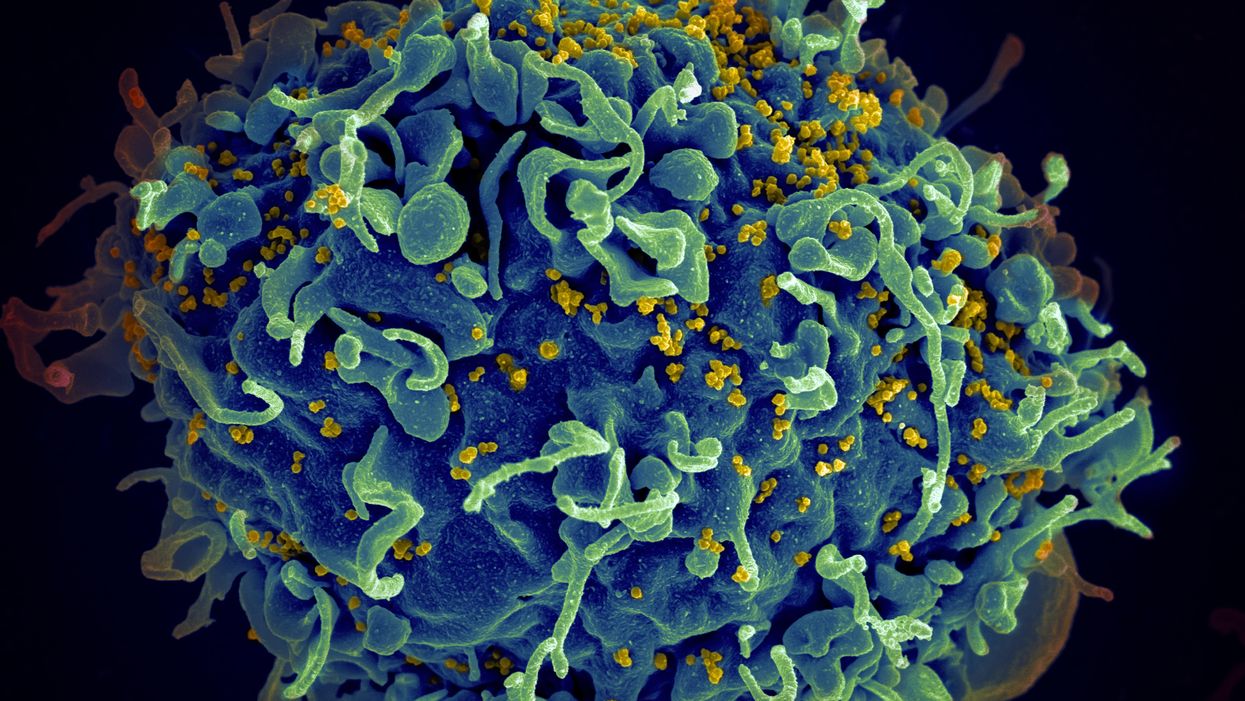
The HIV virus (yellow) infecting a human cell.
Few vaccines have been as complicated—and filled with false starts and crushed hopes—as the development of an HIV vaccine.
While antivirals help HIV-positive patients live longer and reduce viral transmission to virtually nil, these medications must be taken for life, and preventative medications like pre-exposure prophylaxis, known as PrEP, need to be taken every day to be effective. Vaccines, even if they need boosters, would make prevention much easier.
In August, Moderna began human trials for two HIV vaccine candidates based on messenger RNA.
As they have with the Covid-19 pandemic, mRNA vaccines could change the game. The technology could be applied for gene editing therapy, cancer, other infectious diseases—even a universal influenza vaccine.
In the past, three other mRNA vaccines completed phase-2 trials without success. But the easily customizable platforms mean the vaccines can be tweaked better to target HIV as researchers learn more.
Ever since HIV was discovered as the virus causing AIDS, researchers have been searching for a vaccine. But the decades-long journey has so far been fruitless; while some vaccine candidates showed promise in early trials, none of them have worked well among later-stage clinical trials.
There are two main reasons for this: HIV evolves incredibly quickly, and the structure of the virus makes it very difficult to neutralize with antibodies.
"We in HIV medicine have been desperate to find a vaccine that has effectiveness, but this goal has been elusive so far."
"You know the panic that goes on when a new coronavirus variant surfaces?" asked John Moore, professor of microbiology and immunology at Weill Cornell Medicine who has researched HIV vaccines for 25 years. "With HIV, that kind of variation [happens] pretty much every day in everybody who's infected. It's just orders of magnitude more variable a virus."
Vaccines like these usually work by imitating the outer layer of a virus to teach cells how to recognize and fight off the real thing off before it enters the cell. "If you can prevent landing, you can essentially keep the virus out of the cell," said Larry Corey, the former president and director of the Fred Hutchinson Cancer Research Center who helped run a recent trial of a Johnson & Johnson HIV vaccine candidate, which failed its first efficacy trial.
Like the coronavirus, HIV also has a spike protein with a receptor-binding domain—what Moore calls "the notorious RBD"—that could be neutralized with antibodies. But while that target sticks out like a sore thumb in a virus like SARS-CoV-2, in HIV it's buried under a dense shield. That's not the only target for neutralizing the virus, but all of the targets evolve rapidly and are difficult to reach.
"We understand these targets. We know where they are. But it's still proving incredibly difficult to raise antibodies against them by vaccination," Moore said.
In fact, mRNA vaccines for HIV have been under development for years. The Covid vaccines were built on decades of that research. But it's not as simple as building on this momentum, because of how much more complicated HIV is than SARS-CoV-2, researchers said.
"They haven't succeeded because they were not designed appropriately and haven't been able to induce what is necessary for them to induce," Moore said. "The mRNA technology will enable you to produce a lot of antibodies to the HIV envelope, but if they're the wrong antibodies that doesn't solve the problem."
Part of the problem is that the HIV vaccines have to perform better than our own immune systems. Many vaccines are created by imitating how our bodies overcome an infection, but that doesn't happen with HIV. Once you have the virus, you can't fight it off on your own.
"The human immune system actually does not know how to innately cure HIV," Corey said. "We needed to improve upon the human immune system to make it quicker… with Covid. But we have to actually be better than the human immune system" with HIV.
But in the past few years, there have been impressive leaps in understanding how an HIV vaccine might work. Scientists have known for decades that neutralizing antibodies are key for a vaccine. But in 2010 or so, they were able to mimic the HIV spike and understand how antibodies need to disable the virus. "It helps us understand the nature of the problem, but doesn't instantly solve the problem," Moore said. "Without neutralizing antibodies, you don't have a chance."
Because the vaccines need to induce broadly neutralizing antibodies, and because it's very difficult to neutralize the highly variable HIV, any vaccine will likely be a series of shots that teach the immune system to be on the lookout for a variety of potential attacks.
"Each dose is going to have to have a different purpose," Corey said. "And we hope by the end of the third or fourth dose, we will achieve the level of neutralization that we want."
That's not ideal, because each individual component has to be made and tested—and four shots make the vaccine harder to administer.
"You wouldn't even be going down that route, if there was a better alternative," Moore said. "But there isn't a better alternative."
The mRNA platform is exciting because it is easily customizable, which is especially important in fighting against a shapeshifting, complicated virus. And the mRNA platform has shown itself, in the Covid pandemic, to be safe and quick to make. Effective Covid vaccines were comparatively easy to develop, since the coronavirus is easier to battle than HIV. But companies like Moderna are capitalizing on their success to launch other mRNA therapeutics and vaccines, including the HIV trial.
"You can make the vaccine in two months, three months, in a research lab, and not a year—and the cost of that is really less," Corey said. "It gives us a chance to try many more options, if we've got a good response."
In a trial on macaque monkeys, the Moderna vaccine reduced the chances of infection by 85 percent. "The mRNA platform represents a very promising approach for the development of an HIV vaccine in the future," said Dr. Peng Zhang, who is helping lead the trial at the National Institute of Allergy and Infectious Diseases.
Moderna's trial in humans represents "a very exciting possibility for the prevention of HIV infection," Dr. Monica Gandhi, director of the UCSF-Gladstone Center for AIDS Research, said in an email. "We in HIV medicine have been desperate to find a vaccine that has effectiveness, but this goal has been elusive so far."
If a successful HIV vaccine is developed, the series of shots could include an mRNA shot that primes the immune system, followed by protein subunits that generate the necessary antibodies, Moore said.
"I think it's the only thing that's worth doing," he said. "Without something complicated like that, you have no chance of inducing broadly neutralizing antibodies."
"I can't guarantee you that's going to work," Moore added. "It may completely fail. But at least it's got some science behind it."
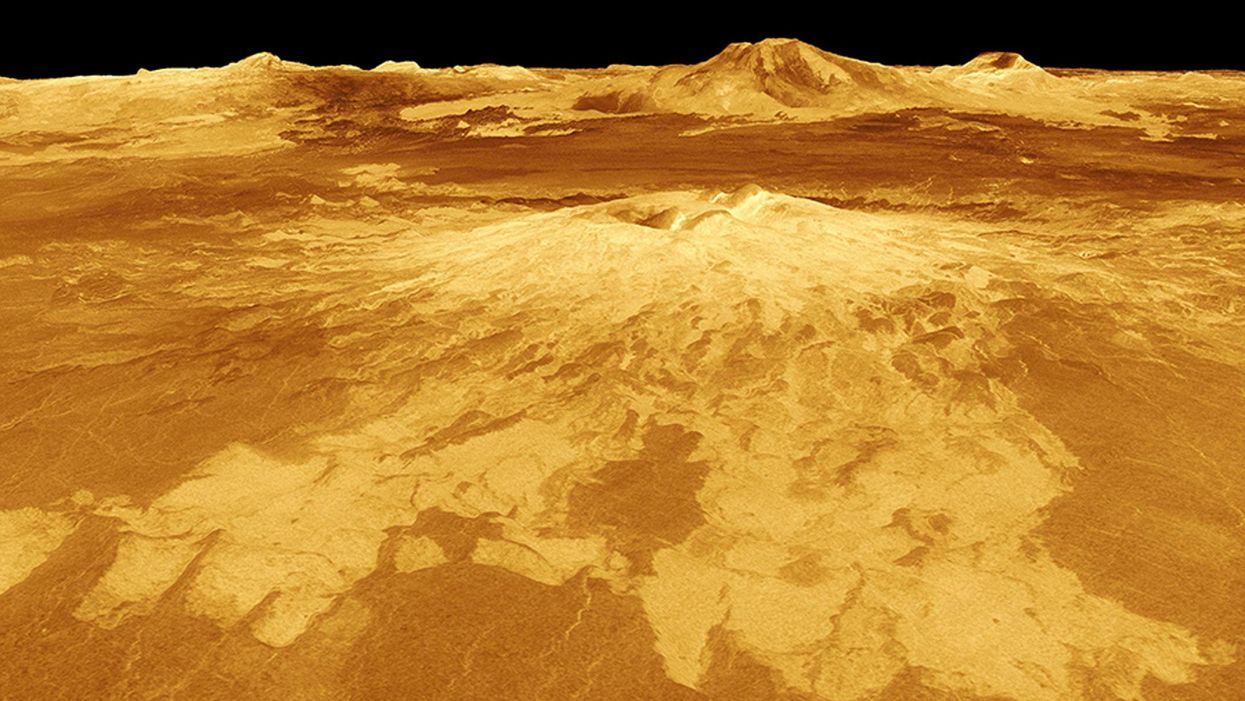
New Podcast: The Lead Scientist for the NASA Mission to Venus
The volcano Sapas Mons is displayed in the center of this computer-generated three-dimensional perspective view of the surface of Venus.
The "Making Sense of Science" podcast features interviews with leading medical and scientific experts about the latest developments and the big ethical and societal questions they raise. This monthly podcast is hosted by journalist Kira Peikoff, founding editor of the award-winning science outlet Leaps.org.
This month, our guest is JPL's Dr. Suzanne Smrekar, who will be pushing the boundaries of knowledge about the planet Venus during the upcoming VERITAS mission set to launch in 2028. Why did Earth's twin planet develop so differently than our own? Could Venus ever have hosted life? What is the bigger purpose for humanity in studying the solar system -- is it purely scientific, or is it also a matter of art and philosophy? Hear Dr. Smrekar discuss all this and more on the latest episode.
Watch the 30-Second Trailer:
Listen to the Episode:
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.