SCOOP: Largest Cryobank in the U.S. to Offer Ancestry Testing

Vanessa Colimorio (left) and Sharon Kochlany (right) at a farm with their four-year-old twin daughters and one-year-old son. The kids share the same sperm donor.
Sharon Kochlany and Vanessa Colimorio's four-year-old twin girls had a classic school assignment recently: make a family tree. They drew themselves and their one-year-old brother branching off from their moms, with aunts, uncles, and grandparents forking off to the sides.
The recently-gained sovereignty of queer families stands to be lost if a consumer DNA test brings a stranger's identity out of the woodwork.
What you don't see in the invisible space between Kochlany and Colimorio, however, is the sperm donor they used to conceive all three children.
To look at a family tree like this is to see in its purest form that kinship can supersede biology—the boundaries of where this family starts and stops are clear to everyone in it, in spite of a third party's genetic involvement. This kind of self-definition has always been synonymous with LGBTQ families, especially those that rely on donor gametes (sperm or eggs) to exist.
But the world around them has changed quite suddenly: The recent consumer DNA testing boom has made it more complicated than ever for families built through reproductive technology—openly, not secretively—to maintain the strong sense of autonomy and privacy that can be crucial for their emotional security. Prospective parents and cryobanks are now mulling how best to bring a new generation of donor-conceived people into this world in a way that leaves open the choice to know more about their ancestry without obliterating an equally important choice: the right not to know about biological relatives.
For queer parents who have long fought for social acceptance, having a biological relationship to their children has been revolutionary, and using an unknown donor as a means to this end especially so. Getting help from a friend often comes with the expectation that the friend will also have social involvement in the family, which some people are comfortable with, but being able to access sperm from an unknown donor—which queer parents have only been able to openly do since the early 1980s—grants them the reproductive autonomy to create families seemingly on their own. That recently-gained sovereignty stands to be lost if a consumer DNA test brings a stranger's identity out of the woodwork.
At the same time, it's natural for donor-conceived people to want to know more about where they come from ethnically, even if they don't want to know the identity of their donor. As a donor-conceived person myself, I know my donor's self-reported ethnicity, but have often wondered how accurate it is.
Opening the Pandora's box of a consumer DNA test as a way to find out has always felt profoundly unappealing to me, however. Many people have accidentally learned they're donor-conceived by unwittingly using these tools, but I already know that about myself going in, and subsequently know I'll be connected to a large web of people whose existence I'm not interested in learning about. In addition to possibly identifying my anonymous donor, his family could also show up, along with any donor-siblings—other people with whom I share a donor. My single lesbian mom is enough for me, and the trade off to learn more about my ethnic ancestry has never seemed worth it.
In 1992, when I was born, no one was planning for how consumer DNA tests might upend or illuminate one's sense of self. But the donor community has always had to stay nimble with balancing privacy concerns and psychological well-being, so it should come as no surprise that figuring out how to do so in 2020 includes finding a way to offer ancestry insight while circumventing consumer DNA tests.
A New Paradigm
This is the rationale behind unprecedented industry news that LeapsMag can exclusively break: Within the next few weeks, California Cryobank, the largest cryobank in the country, will begin offering genetically-verified ancestry information on the free public part of every donor's anonymous profile in its database, something no other cryobanks yet offer (an exact launch date was not available at the time of publication). Currently, California Cryobank's donor profiles include a short self-reported list that might merely say, "Ancestry: German, Lebanese, Scottish."
The new information will be a report in pie chart form that details exactly what percentages of a donor's DNA come from up to 26 ethnicities—it's analogous to, but on a smaller scale than, the format offered by consumer DNA testing companies, and uses the same base technology that looks for single nucleotide polymorphisms in DNA that are associated with specific ethnicities. But crucially, because the donor takes the DNA test through California Cryobank, not a consumer-facing service, the information is not connected in a network to anyone else's DNA test. It's also taken before any offspring exist so there's no chance of revealing a donor-conceived person's identity this way.
Later, when a donor-conceived person is born, grows up, and wants information about their ethnicity from the donor side, all they need is their donor's anonymous ID number to look it up. The donor-conceived person never takes a genetic test, and therefore also can't accidentally find donor siblings this way. People who want to be connected to donor siblings can use a sibling registry where other people who want to be found share donor ID numbers and look for matches (this is something that's been available for decades, and remains so).
"With genetic testing, you have no control over who reaches out to you, and at what point in your life."
California Cryobank will require all new donors to consent to this extra level of genetic testing, setting a new standard for what information prospective parents and donor-conceived people can expect to have. In the immediate, this information will be most useful for prospective parents looking for donors with specific backgrounds, possibly ones similar to their own.
It's a solution that was actually hiding in plain sight. Two years ago, California Cryobank's partner Sema4, the company handling the genetic carrier testing that's used to screen for heritable diseases, started analyzing ethnic data in its samples. That extra information was being collected because it can help calculate a more accurate assessment of genetic risks that run in certain populations—like Ashkenazi Jews and Tay Sachs disease—than relying on oral family histories. Shortly after a plan to start collecting these extra data, Jamie Shamonki, chief medical officer of California Cryobank, realized the companies would be sitting on a goldmine for a different reason.
"I didn't want to use one of these genetic testing companies like Ancestry to accomplish this," says Shamonki. "The whole thing we're trying to accomplish is also privacy."
Consumer-facing DNA testing companies are not HIPAA compliant (whereas Sema4, which isn't direct-to-consumer, is HIPAA compliant), which means there are no legal privacy protections covering people who add their DNA to these databases. Although some companies, like 23andMe, allow users to opt-out of being connected with genetic relatives, the language can be confusing to navigate, requires a high level of knowledge and self-advocacy on the user's part, and, as an opt-out system, is not set up to protect the user from unwanted information by default; many unwittingly walk right into such information as a result.
Additionally, because consumer-facing DNA testing companies operate outside the legal purview that applies to other health care entities, like hospitals, even a person who does opt-out of being linked to genetic relatives is not protected in perpetuity from being re-identified in the future by a change in company policy. The safest option for people with privacy concerns is to stay out of these databases altogether.
For California Cryobank, the new information about donor heritage won't retroactively be added to older profiles in the system, so donor-conceived people who already exist won't benefit from the ancestry tool, but it'll be the new standard going forward. The company has about 500 available donors right now, many of which have been in their registry for a while; about 100 of those donors, all new, will have this ancestry data on their profiles.
Shamonki says it has taken about two years to get to the point of publicly including ancestry information on a donor's profile because it takes about nine months of medical and psychological screening for a donor to go from walking through the door to being added to their registry. The company wanted to wait to launch until it could offer this information for a significant number of donors. As more new donors come online under the new protocol, the number with ancestry information on their profiles will go up.
For Parents: An Unexpected Complication
While this change will no doubt be welcome progress for LGBTQ families contemplating parenthood, it'll never be possible to put this entire new order back in the box. What are such families who already have donor-conceived children losing in today's world of widespread consumer genetic testing?
Kochlany and Colimorio's twins aren't themselves much older than the moment at-home DNA testing really started to take off. They were born in 2015, and two years later the industry saw its most significant spike. By now, more than 26 million people's DNA is in databases like 23andMe and Ancestry; as a result, it's estimated that within a year, 90 percent of Americans of European descent will be identifiable through these consumer databases, by way of genetic third cousins, even if they didn't want to be found and never took the test themselves. This was the principle behind solving the Golden State Killer cold case.
The waning of privacy through consumer DNA testing fundamentally clashes with the priorities of the cyrobank industry, which has long sought to protect the privacy of donor-conceived people, even as open identification became standard. Since the 1980s, donors have been able to allow their identity to be released to any offspring who is at least 18 and wants the information. Lesbian moms pushed for this option early on so their children—who would obviously know they couldn't possibly be the biological product of both parents—would never feel cut off from the chance to know more about themselves. But importantly, the openness is not a two-way street: the donors can't ever ask for the identities of their offspring. It's the latter that consumer DNA testing really puts at stake.
"23andMe basically created the possibility that there will be donors who will have contact with their donor-conceived children, and that's not something that I think the donor community is comfortable with," says I. Glenn Cohen, director of Harvard Law School's Center for Health Law Policy, Biotechnology & Bioethics. "That's about the donor's autonomy, not the rearing parents' autonomy, or the donor-conceived child's autonomy."
Kochlany and Colimorio have an open identification donor and fully support their children reaching out to California Cryobank to get more information about him if they want to when they're 18, but having a singular name revealed isn't the same thing as having contact, nor is it the same thing as revealing a web of dozens of extended genetic relations. Their concern now is that if their kids participate in genetic testing, a stranger—someone they're careful to refer to as only "the donor" and never "dad"—will reach out to the children to begin some kind of relationship. They know other people who are contemplating giving their children DNA tests, and feel staunchly that it wouldn't be right for their family.
"With genetic testing, you have no control over who reaches out to you, and at what point in your life," Kochlany says. "[People] reaching out and trying to say, 'Hey I know who your dad is' throws a curveball. It's like, 'Wait, I never thought I had a dad.' It might put insecurities in their minds."
"We want them to have the opportunity to choose whether or not they want to reach out," Colimorio adds.
Kochlany says that when their twins are old enough to start asking questions, she and Colimorio plan to frame it like this: "The donor was kind of like a technology that helped us make you a person, and make sure that you exist," she says, role playing a conversation with their kids. "But it's not necessarily that you're looking to this person [for] support or love, or because you're missing a piece."
It's a line in the sand that's present even for couples still far off from conceiving. When Mallory Schwartz, a film and TV producer in Los Angeles, and Lauren Pietra, a marriage and family therapy associate (and Shamonki's step-daughter), talk about getting married someday, it's a package deal with talking about how they'll approach having kids. They feel there are too many variables and choices to make around family planning as a same-sex couple these days to not have those conversations simultaneously. Consumer DNA databases are already on their minds.
"It frustrates me that the DNA databases are just totally unregulated," says Schwartz. "I hope they are by the time we do this. I think everyone deserves a right to privacy when making your family [using a sperm donor]."
"I wouldn't want to create a world where people who are donor-conceived feel like they can't participate in this technology because they're trying to shut out [other] information."
On the prospect of having a donor relation pop up non-consensually for a future child, Pietra says, "I don't like it. It would be really disappointing if the child didn't want [contact], and unfortunately they're on the receiving end."
You can see how important preserving the right to keep this door closed is when you look at what's going on at The Sperm Bank of California. This pioneering cryobank was the first in the world to openly serve LGBTQ people and single women, and also the first to offer the open identification option when it opened in 1982, but not as many people are asking for their donor's identity as expected.
"We're finding a third of young people are coming forward for their donor's identity," says Alice Ruby, executive director. "We thought it would be a higher number." Viewed the other way, two-thirds of the donor-conceived people who could ethically get their donor's identity through The Sperm Bank of California are not asking the cryobank for it.
Ruby says that part of what historically made an open identification program appealing, rather than invasive or nerve-wracking, is how rigidly it's always been formatted around mutual consent, and protects against surprises for all parties. Those [donor-conceived people] who wanted more information were never barred from it, while those who wanted to remain in the dark could. No one group's wish eclipsed the other's. The potential breakdown of a system built around consent, expectations, and respect for privacy is why unregulated consumer DNA testing is most concerning to her as a path for connecting with genetic relatives.
For the last few decades in cryobanks around the world, the largest cohort of people seeking out donor sperm has been lesbian couples, followed by single women. For infertile heterosexual couples, the smallest client demographic, Ruby says donor sperm offers a solution to a medical problem, but in contrast, it historically "provided the ability for [lesbian] couples and single moms to have some reproductive autonomy." Yes, it was still a solution to a biological problem, but it was also a solution to a social one.
The Sperm Bank of California updated its registration forms to include language urging parents, donor-conceived people, and donors not to use consumer DNA tests, and to go through the cryobank if they, understandably, want to learn more about who they're connected to. But truthfully, there's not much else cryobanks can do to protect clients on any side of the donor transaction from surprise contact right now—especially not from relatives of the donor who may not even know someone in their family has donated sperm.
A Tricky Position
Personally, I've known I was donor-conceived from day one. It has never been a source of confusion, angst, or curiosity, and in fact has never loomed particularly large for me in any way. I see it merely as a type of reproductive technology—on par with in vitro fertilization—that enabled me to exist, and, now that I do exist, is irrelevant. Being confronted with my donor's identity or any donor siblings would make this fact of my conception bigger than I need it to be, as an adult with a full-blown identity derived from all of my other life experiences. But I still wonder about the minutiae of my ethnicity in much the same way as anyone else who wonders, and feel there's no safe way for me to find out without relinquishing some of my existential independence.
"People obviously want to participate in 23andMe and Ancestry because they're interested in knowing more about themselves," says Shamonki. "I wouldn't want to create a world where people who are donor-conceived feel like they can't participate in this technology because they're trying to shut out [other] information."
After all, it was the allure of that exact conceit—knowing more about oneself—that seemed to magnetically draw in millions of people to these tools in the first place. It's an experience that clearly taps into a population-wide psychic need, even—perhaps especially—if one's origins are a mystery.
These doctors have a heart for recycling
In the U.S. and Europe, it is illegal to reuse pacemakers and other implants. Therefore, cardiologists export them to the global South where they save the lives of people of all ages.
This is part 3 of a three part series on a new generation of doctors leading the charge to make the health care industry more sustainable - for the benefit of their patients and the planet. Read part 1 here and part 2 here.
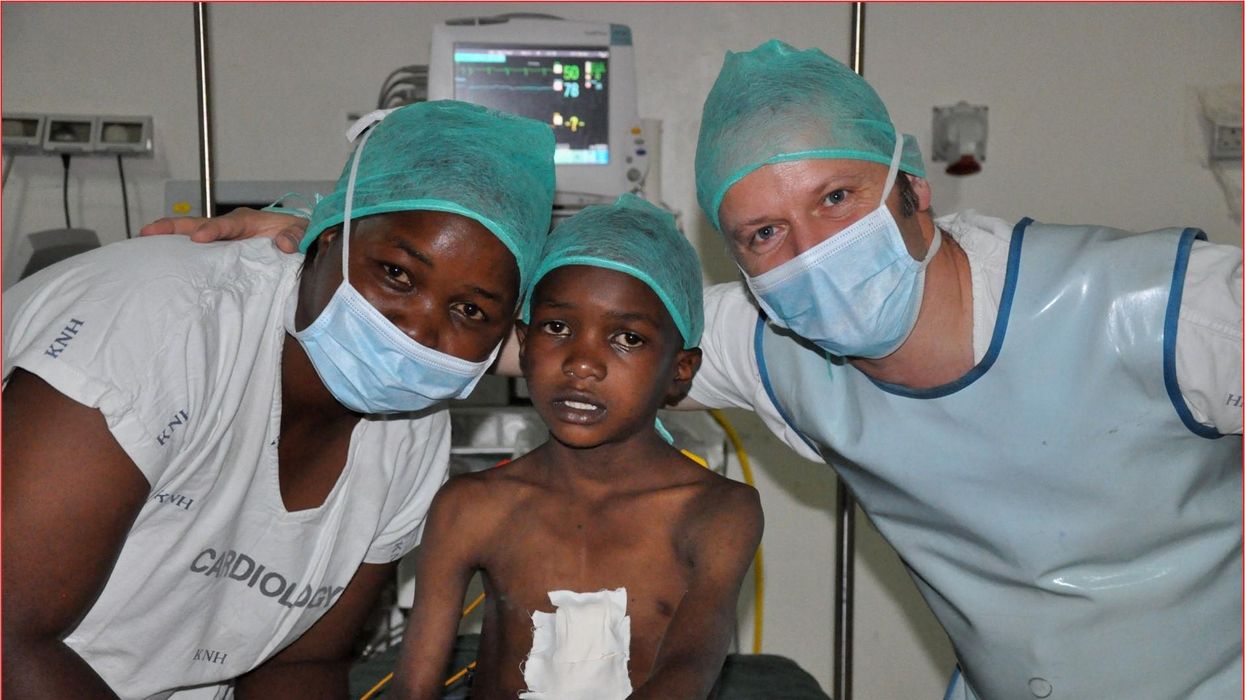
One could say that over 400 people owe their life to the fact that Carsten Israel fell in love. Twenty years ago, as a young doctor in Frankfurt, Germany, he began to court an au pair from Kenya, Elisabeth, his now-wife of 13 years with whom he has three children. When the couple started visiting her parents in Kenya, Israel wanted to check out the local hospitals, “just out of professional curiosity,“ says the cardiologist, who is currently the head doctor at the Clinic for Interior Medicine in Bielefeld. “I was completely shocked.“
Often he observed there were no doctors in the E.R.s, and hte nurses could render only basic first aid. “When somebody fell into a coma, they fell into a coma,“ Israel remembers. “There weren’t even any defibrillators to restart a patient’s heart,” while defibrillators are standard equipment in most clinics in the U.S. and Europe as lifesaving devices. When Israel finally visited the largest and most modern hospital in Nairobi, he found it better equipped but he learned that its services were only available to patients who could afford them. The cardiologist there had a drawer full of petitions from patients with heart ailments who couldn’t afford lifesaving surgery. Even two decades ago, a pacemaker cost $5,000 in Kenya, which made it unaffordable for most Kenyans who earn an average of $600 per month.
Since 2003, Israel and a team of two doctors and two nurses visit Kenya and Zambia once or twice a year to implant German pacemakers for free. Notably, the pacemakers and defibrillators Israel exports to Africa would end up in the landfill in Germany. Clinics have to pay for specialized services to dispose of this medical equipment. “In Germany, I could go to jail if I used a defibrillator that is one day past its expiration date,“ Israel says, “but in Kenya, people don’t have the money for the cheapest model. What nonsense to throw this precious medical equipment away while people in poorer countries die because they desperately need it.“
Israel works at the breakpoint between the laws in a wealthy country like Germany and the reality in the global South. The U.S. and most European countries have strict laws that ban the reuse of medical implants and enforce strict expiration dates for medical equipment. “But if a pacemaker is a few days past its expiration date, it still works perfectly fine,“ Israel says. “And it also happens that we implant a pacemaker and five months later it turns out that the patient needs a different kind. Then we replace it and we’d have to trash the first one in Germany, though it could easily run another 12 years.“
“If we get this right, we have lots of devices we can implant, hips and knees, etcetera. Where this will lead is limitless," says Eva Kline Rogers, the program coordinator for My Heart, Your Heart.
Israel has been collecting donations of pacemakers and defibrillators from manufacturers but also from other doctors and from funeral homes for his nonprofit Pacemakers for East Africa since 2003. Most funeral homes in the U.S. and Europe are legally obliged to remove pacemakers from the dead before cremation. “Most pacemakers survive their owners,“ says Israel. He sterilizes the pacemakers and finds them a new life in East Africa. Studies show that reused pacemakers carry no greater risk for the patients than new ones.
In the U.S., University of Michigan professor Thomas Crawford heads up a similar initiative, My Heart, Your Heart. “Each year 1 to 2 million individuals worldwide die due to a lack of access to pacemakers and defibrillators,” the organization notes on its website. The nonprofit was founded in 2009, but it took four years for the doctors to get permission from the FDA to export pacemakers. Since receiving permission, the organization has sent dozens of devices to the Philippines, Haiti, Venezuela, Kenya, Sierra Leone and Ukraine. “We were the first doctors ever to implant a pacemaker in Sierra Leone in 2018,” says Crawford, who has traveled extensively to most of the recipient countries.
Even individuals can donate their pacemakers; the organization offers a prepaid envelope. “My mother recently passed and she donated her device,” says Tina Alexandris-Souphis, one of the doctors at University of Michigan who collaborates on My Heart, Your Heart. The organization works with World Medical Relief and the U.K. based charity Pace4Life to maintain a registry of the most urgent patients and send devices to where they are needed the most.
My Heart, Your Heart is also conducting a randomized controlled trial to provide further evidence that reused pacemakers pose no additional risk. “Our vision is that we establish this is safe and create a blueprint for organizations around the world to safely reuse these devices instead of them being thrown in the trash,” says Eva Kline Rogers, the program’s coordinator. “If we get this right, we have lots of devices we can implant, hips and knees, etc. Where this will lead is limitless.” She points out that in addition to receiving the donated devices, the doctors in the global South also benefit from the expertise of renowned cardiologists, such as Crawford, who sometimes advise them in complex cases.
And Adrian Baranchuk, a Canadian doctor at the Kingston General Hospital at the Queen’s University, regularly travels through South America with his “cardiology van” to help villagers in remote areas with heart problems.
Israel says that he’s been accused of racism, in thinking that these pacemakers are suitable for those in the global South - many of whom are people of color - even though officials in wealthier countries consider them to be trash. The cardiologist counters such criticism with stories about desperate need of his patients. At his first medical visit to Nairobi that he organized with a local cardiologist, six patients were waiting for him. “In Germany, they would all be considered emergencies,” Israel says. One eighty-year old grandmother had a heartrate of 18. “I’ve never before seen anything like this,” Israel exclaims. “At first I thought I couldn’t find her pulse before I realized that her heart was only beating once every three seconds.” After the surgery, she got up, dressed herself and hurriedly packed her bag, explaining she had a ton of work to accomplish. Her family was in disbelief, Israel says. “They told me she had been bedridden for five years because as soon as she tried to get up she would faint.”

Israel has been accused of racism, in thinking that these pacemakers are suitable for those in the global South even though they're considered to be trash by officials in wealthier countries. The cardiologist counters such criticism with stories about desperate need of his patients.
Carsten Israel
The hospital in Nairobi where Israel conducts the surgeries, charges patients $200 for the use of its facilities. If patients can’t afford that sum, Israel will pay it from the funds of his nonprofit. For some people, $200 far exceeds their resources. Once, a family from the extremely poor Northern region of Kenya told him they couldn’t afford the $3 for the bus ride to Nairobi. Israel suspected this was a pretense because they were afraid of the surgery and agreed to reimburse the $3, “but when they came, they were wearing rags and were so rail-thin, I understood that they really needed every cent they had for food.”
Israel is a renowned cardiologists in Germany. And yet, he considers his work in East Africa to be particularly meaningful. “Generally, most patients in Germany will get the treatment they need,” he says, “and I never before experienced that people have an illness that is easily curable but simply won’t be treated.” He also feels a heavy responsibility. Many patients have his personal cell phone and call him when they have problems or good news about how they’re doing.
Some of those progress reports come much faster than in Israel’s home country. Before he implanted a pacemaker in a tall Massai in Kenya, the man had been picked on by his family because he wouldn’t help much with the hard work on the family peanut farm. “When I examined him, he had a pulse of 40,” Israel says. “It’s a miracle he was even standing upright, let alone hauling heavy bags.” After the surgery, Israel advised his patient to stay the night for observation, but the patient couldn’t wait to leave. Two hours later, he returned, covered in sweat. He’d been running sprints with his brothers to test the new device. Israel shakes his head. In Germany, it would be unthinkable for a patient to engage in athletics immediately after surgery. But the patient was exuberant: “I was the fastest!”
The success stories are notable partly because the challenges remain so steep. In Zambia, for instance, there is a single cardiologist; she determined to become one after losing her younger sister to an easily curable heart disease. Often, the hospitals not only lack pacemakers but also sterile surgery equipment, antibiotics and other essential material. Therefore, Israel and his team import everything they need for the surgeries, including medication. If necessary, they improvise. “I’ve done surgery with a desk lamp hanging from the ceiling by threads,” Israel says. He already knows that he will need to return to Kenya in six months to replace the pacemaker of one of his patients and replace the batteries in others. If he doesn’t travel, lives are at risk.These technologies may help more animals and plants survive climate change
As the climate changes, the ripples will reach everywhere. Better data is needed for both plants and animals, and scientists are looking for genes that could allow crops to survive.
This article originally appeared in One Health/One Planet, a single-issue magazine that explores how climate change and other environmental shifts are making us more vulnerable to infectious diseases by land and by sea - and how scientists are working on solutions.
Along the west coast of South Florida and the Keys, Florida Bay is a nursery for young Caribbean spiny lobsters, a favorite local delicacy. Growing up in small shallow basins, they are especially vulnerable to warmer, more saline water. Climate change has brought tidal floods, bleached coral reefs and toxic algal blooms to the state, and since the 1990s, the population of the Caribbean spiny lobster has dropped some 20 percent, diminishing an important food for snapper, grouper, and herons, as well as people. In 1999, marine ecologist Donald Behringer discovered the first known virus among lobsters, Panulirus argus virus—about a quarter of juveniles die from it before they mature.
“When the water is warm PaV1 progresses much more quickly,” says Behringer, who is based at the Emerging Pathogens Institute at the University of Florida in Gainesville.
Caribbean spiny lobsters are only one example of many species that are struggling in the era of climate change, both at sea and on land. As the oceans heat up, absorbing greenhouse gases and growing more acidic, marine diseases are emerging at an accelerated rate. Marine creatures are migrating to new places, and carrying pathogens with them. The latest grim report in the journal Science, states that if global warming continues at the current rate, the extinction of marine species will rival the Permian–Triassic extinction, sometimes called the “Great Dying,” when volcanoes poisoned the air and wiped out as much as 90 percent of all marine life 252 million years ago.
Similarly, on land, climate change has exposed wildlife, trees and crops to new or more virulent pathogens. Warming environments allow fungi, bacteria, viruses and infectious worms to proliferate in new species and locations or become more virulent. One paper modeling records of nearly 1,400 wildlife species projects that parasites will double by 2070 in the far north and in high-altitude places. Right now, we are seeing the effects most clearly on the fringes—along the coasts, up north and high in the mountains—but as the climate continues changing, the ripples will reach everywhere.
Few species are spared
On the Hawaiian Islands, mosquitoes are killing more songbirds. The dusky gray akikiki of Kauai and the chartreuse-yellow kiwikiu of Maui could vanish in two years, under assault from mosquitoes bearing avian malaria, according to a University of Hawaiʻi 2022 report. Previously, the birds could escape infection by roosting high in the cold mountains, where the pests couldn’t thrive, but climate change expanded the range of the mosquito and narrowed theirs.
Likewise, as more midge larvae survive over warm winters and breed better during drier summers, they bite more white-tailed deer, spreading often-fatal epizootic hemorrhagic disease. Especially in northern regions of the globe, climate change brings the threat of midges carrying blue tongue disease, a virus, to sheep and other animals. Tick-borne diseases like encephalitis and Lyme disease may become a greater threat to animals and perhaps humans.
"If you put all your eggs in one basket and then a pest comes a long, then you are more vulnerable to those risks," says Mehroad Ehsani, managing director of the food initiative in Africa for the Rockefeller Foundation. "Research is needed on resilient, climate smart, regenerative agriculture."
In the “thermal mismatch” theory of wildlife disease, cold-adapted species are at greater risk when their habitats warm, and warm-adapted species suffer when their habitats cool. Mammals can adjust their body temperature to adapt to some extent. Amphibians, fish and insects that cannot regulate body temperatures may be at greater risk. Many scientists see amphibians, especially, as canaries in the coalmine, signaling toxicity.
Early melting ice can foster disease. Climate models predict that the spring thaw will come ever-earlier in the lakes of the French Pyrenees, for instance, which traditionally stayed frozen for up to half the year. The tadpoles of the midwife toad live under the ice, where they are often infected with amphibian chytrid fungus. When a seven-year study tracked the virus in three species of amphibians in Pyrenees’s Lac Arlet, the research team found that, the earlier the spring thaw arrived, the more infection rates rose in common toads— , while remaining high among the midwife toads. But the team made another sad discovery: with early thaws, the common frog, which was thought to be free of the disease in Europe, also became infected with the fungus and died in large numbers.
Changing habitats affect animal behavior. Normally, spiny lobsters rely on chemical cues to avoid predators and sick lobsters. New conditions may be hampering their ability to “social distance”—which may help PaV1 spread, Behringer’s research suggests. Migration brings other risks. In April 2022, an international team led by scientists at Georgetown University announced the first comprehensive overview, published in the journal Nature, of how wild mammals under pressure from a changing climate may mingle with new populations and species—giving viruses a deadly opportunity to jump between hosts. Droughts, for example, will push animals to congregate at the few places where water remains.
Plants face threats also. At the timberline of the cold, windy, snowy mountains of the U.S. west, whitebark pine forests are facing a double threat, from white pine blister rust, a fungal disease, and multiplying pine beetles. “If we do nothing, we will lose the species,” says Robert Keane, a research ecologist for the U.S. Forest Service, based in Missoula, Montana. That would be a huge shift, he explains: “It’s a keystone species. There are over 110 animals that depend on it, many insects, and hundreds of plants.” In the past, beetle larvae would take two years to complete their lifecycle, and many died in frost. “With climate change, we're seeing more and more beetles survive, and sometimes the beetle can complete its lifecycle in one year,” he says.
Quintessential crops are under threat too
As some pathogens move north and new ones develop, they pose novel threats to the crops humans depend upon. This is already happening to wheat, coffee, bananas and maize.
Breeding against wheat stem rust, a fungus long linked to famine, was a key success in the mid-20th century Green Revolution, which brought higher yields around the world. In 2013, wheat stem rust reemerged in Germany after decades of absence. It ravaged both bread and durum wheat in Sicily in 2016 and has spread as far as England and Ireland. Wheat blast disease, caused by a different fungus, appeared in Bangladesh in 2016, and spread to India, the world’s second largest producer of wheat.
Insects, moths, worms, and coffee leaf rust—a fungus now found in all coffee-growing countries—threaten the livelihoods of millions of people who grow coffee, as well as everybody’s cup of joe. More heat, more intense rain, and higher humidity have allowed coffee leaf rust to cycle more rapidly. It has grown exponentially, overcoming the agricultural chemicals that once kept it under control.
To identify new diseases and fine-tune crops for resistance, scientists are increasingly relying on genomic tools.
Tar spot, a fungus native to Latin America that can cut corn production in half, has emerged in highland areas of Central Mexico and parts of the U.S.. Meanwhile, maize lethal necrosis disease has spread to multiple countries in Africa, notes Mehrdad Ehsani, Managing Director for the Food Initiative in Africa of the Rockefeller Foundation. The Cavendish banana, which most people eat today, was bred to be resistant to the fungus Panama 1. Now a new fungus, Panama 4, has emerged on every continent–including areas of Latin America that rely on the Cavendish for their income, reported a recent story in the Guardian. New threats are poised to emerge. Potato growers in the Andes Mountains have been shielded from disease because of colder weather at high altitude, but temperature fluxes and warming weather are expected to make this crop vulnerable to potato blight, found plant pathologist Erica Goss, at the Emerging Pathogens Institute.
Science seeks solutions
To protect food supplies in the era of climate change, scientists are calling for integrated global surveillance systems for crop disease outbreaks. “You can imagine that a new crop variety that is drought-tolerant could be susceptible to a pathogen that previous varieties had some resistance against,” Goss says. “Or a country suffers from a calamitous weather event, has to import seed from another country, and that seed is contaminated with a new pathogen or more virulent strain of an existing pathogen.” Researchers at the John Innes Center in Norwich and Aarhus University in Denmark have established ways to monitor wheat rust, for example.
Better data is essential, for both plants and animals. Historically, models of climate change predicted effects on plant pathogens based on mean temperatures, and scientists tracked plant responses to constant temperatures, explains Goss. “There is a need for more realistic tests of the effects of changing temperatures, particularly changes in daily high and low temperatures on pathogens,” she says.
To identify new diseases and fine-tune crops for resistance, scientists are increasingly relying on genomic tools. Goss suggests factoring the impact of climate change into those tools. Genomic efforts to select soft red winter wheat that is resistant to Fusarium head blight (FHB), a fungus that plagues farmers in the Southeastern U.S., have had early success. But temperature changes introduce a new factor.
A fundamental solution would be to bring back diversification in farming, says Ehsani. Thousands of plant species are edible, yet we rely on a handful. Wild relatives of domesticated crops are a store of possibly useful genes that may confer resistance to disease. The same is true for livestock. “If you put all your eggs in one basket and then a pest comes along, then you are more vulnerable to those risks. Research is needed on resilient, climate smart, regenerative agriculture,” Ehsani says.
Jonathan Sleeman, director of the U.S. Geological Survey National Wildlife Health Center, has called for data on wildlife health to be systematically collected and integrated with climate and other variables because more comprehensive data will result in better preventive action. “We have focused on detecting diseases,” he says, but a more holistic strategy would apply human public health concepts to assuring animal wellbeing. (For example, one study asked experts to draw a diagram of relationships of all the factors affecting the health of a particular group of caribou.) We must not take the health of plants and animals for granted, because their vulnerability inevitably affects us too, Sleeman says. “We need to improve the resilience of wildlife populations so they can withstand the impact of climate change.”