Should Genetic Information About Mental Health Affect Civil Court Cases?

A rendering of DNA with a judge's gavel.
Imagine this scenario: A couple is involved in a heated custody dispute over their only child. As part of the effort to make the case of being a better guardian, one parent goes on a "genetic fishing expedition": this parent obtains a DNA sample from the other parent with the hope that such data will identify some genetic predisposition to a psychiatric condition (e.g., schizophrenia) and tilt the judge's custody decision in his or her favor.
As knowledge of psychiatric genetics is growing, it is likely to be introduced in civil cases, such as child custody disputes and education-related cases, raising a tangle of ethical and legal questions.
This is an example of how "behavioral genetic evidence" -- an umbrella term for information gathered from family history and genetic testing about pathological behaviors, including psychiatric conditions—may in the future be brought by litigants in court proceedings. Such evidence has been discussed primarily when criminal defendants sought to introduce it to make the claim that they are not responsible for their behavior or to justify their request for reduced sentencing and more lenient punishment.
However, civil cases are an emerging frontier for behavioral genetic evidence. It has already been introduced in tort litigation, such as personal injury claims, and as knowledge of psychiatric genetics is growing, it is further likely to be introduced in other civil cases, such as child custody disputes and education-related cases. But the introduction of such evidence raises a tangle of ethical and legal questions that civil courts will need to address. For example: how should such data be obtained? Who should get to present it and under what circumstances? And does the use of such evidence fit with the purposes of administering justice?
How Did We Get Here?
That behavioral genetic evidence is entering courts is unsurprising. Scientific evidence is a common feature of judicial proceedings, and genetic information may reveal relevant findings. For example, genetic evidence may elucidate whether a child's medical condition is due to genetic causes or medical malpractice, and it has been routinely used to identify alleged offenders or putative fathers. But behavioral genetic evidence is different from such other genetic data – it is shades of gray, instead of black and white.
Although efforts to understand the nature and origins of human behavior are ongoing, existing and likely future knowledge about behavioral genetics is limited. Behavioral disorders are highly complex and diverse. They commonly involve not one but multiple genes, each with a relatively small effect. They are impacted by many, yet unknown, interactions between genes, familial, and environmental factors such as poverty and childhood adversity.
And a specific gene variant may be associated with more than one behavioral disorder and be manifested with significantly different symptoms. Thus, biomarkers about "predispositions" for behavioral disorders cannot generally provide a diagnosis or an accurate estimate of whether, when, and at what severity a behavioral disorder will occur. And, unlike genetic testing that can confirm litigants' identity with 99.99% probability, behavioral genetic evidence is far more speculative.
Genetic theft raises questions about whose behavioral data are being obtained, by whom, and with what authority.
Whether judges, jurors, and other experts understand the nuances of behavioral genetics is unclear. Many people over-estimate the deterministic nature of genetics, and under-estimate the role of environments, especially with regards to mental health status. The U.S. individualistic culture of self-reliance and independence may further tilt the judicial scales because litigants in civil courts may be unjustly blamed for their "bad genes" while structural and societal determinants that lead to poor behavioral outcomes are ignored.
These concerns were recently captured in the Netflix series "13 Reasons Why," depicting a negligence lawsuit against a school brought by parents of a high-school student there (Hannah) who committed suicide. The legal tides shifted from the school's negligence in tolerating a culture of bullying to parental responsibility once cross-examination of Hannah's mother revealed a family history of anxiety, and the possibility that Hannah had a predisposition for mental illness, which (arguably) required therapy even in the absence of clear symptoms.
Where Is This Going?
The concerns are exacerbated given the ways in which behavioral genetic evidence may come to court in the future. One way is through "genetic theft," where genetic evidence is obtained from deserted property, such as soft-drink cans. This method is often used for identification purposes such as criminal and paternity proceedings, and it will likely expand to behavioral genetic data once available through "home kits" that are offered by direct-to-consumer companies.
Genetic theft raises questions about whose behavioral data are being obtained, by whom, and with what authority. In the scenario of child-custody dispute, for example, the sequencing of the other parent's DNA will necessarily intrude on the privacy of that parent, even as the scientific value of such information is limited. A parent on a "genetic fishing expedition" can also secretly sequence their child for psychiatric genetic predispositions, arguably, in order to take preventative measures to reduce the child's risk for developing a behavioral disorder. But should a parent be allowed to sequence the child without the other parent's consent, or regardless of whether the results will provide medical benefits to the child?
Similarly, although schools are required, and may be held accountable for failing to identify children with behavioral disabilities and to evaluate their educational needs, some parents may decline their child's evaluation by mental health professionals. Should schools secretly obtain a sample and sequence children for behavioral disorders, regardless of parental consent? My study of parents found that the overwhelming majority opposed imposed genetic testing by school authorities. But should parental preference or the child's best interests be the determinative factor? Alternatively, could schools use secretly obtained genetic data as a defense that they are fulfilling the child-find requirement under the law?
The stigma associated with behavioral disorders may intimidate some people enough that they back down from just claims.
In general, samples obtained through genetic theft may not meet the legal requirements for admissible evidence, and as these examples suggest, they also involve privacy infringement that may be unjustified in civil litigation. But their introduction in courts may influence judicial proceedings. It is hard to disregard such evidence even if decision-makers are told to ignore it.
The costs associated with genetic testing may further intensify power differences among litigants. Because not everyone can pay for DNA sequencing, there is a risk that those with more resources will be "better off" in court proceedings. Simultaneously, the stigma associated with behavioral disorders may intimidate some people enough that they back down from just claims. For example, a good parent may give up a custody claim to avoid disclosure of his or her genetic predispositions for psychiatric conditions. Regulating this area of law is necessary to prevent misuses of scientific technologies and to ensure that powerful actors do not have an unfair advantage over weaker litigants.
Behavioral genetic evidence may also enter the courts through subpoena of data obtained in clinical, research or other commercial genomic settings such as ancestry testing (similar to the genealogy database recently used to identify the Golden State Killer). Although court orders to testify or present evidence are common, their use for obtaining behavioral genetic evidence raises concerns.
One worry is that it may be over-intrusive. Because behavioral genetics are heritable, such data may reveal information not only about the individual litigant but also about other family members who may subsequently be stigmatized as well. And, even if we assume that many people may be willing for their data in genomic databases to be used to identify relatives who committed crimes (e.g., a rapist or a murderer), we can't assume the same for civil litigation, where the public interest in disclosure is far weaker.
Another worry is that it may deter people from participating in activities that society has an interest in advancing, including medical treatment involving genetic testing and genomic research. To address this concern, existing policy provides expanded privacy protections for NIH-funded genomic research by automatically issuing a Certificate of Confidentiality that prohibits disclosure of identifiable information in any Federal, State, or local civil, criminal, and other legal proceedings.
But this policy has limitations. It applies only to specific research settings and does not cover non-NIH funded research or clinical testing. The Certificate's protections can also be waived under certain circumstances. People who volunteer to participate in non-NIH-funded genomic research for the public good may thus find themselves worse-off if embroiled in legal proceedings.
Consider the following: if a parent in a child custody dispute had participated in a genetic study on schizophrenia years earlier, should the genetic results be subpoenaed by the court – and weaponized by the other parent? Public policy should aim to reduce the risks for such individuals. The end of obtaining behavioral genetic evidence cannot, and should not, always justify the means.
Meet Dr. Renee Wegrzyn, the first Director of President Biden's new health agency, ARPA-H
Today's podcast guest, Dr. Renee Wegrzyn, directs ARPA-H, a new agency formed last year to spearhead health innovations. Time will tell if ARPA-H will produce advances on the level of its fellow agency, DARPA.
In today’s podcast episode, I talk with Renee Wegrzyn, appointed by President Biden as the first director of a health agency created last year, the Advanced Research Projects Agency for Health, or ARPA-H. It’s inspired by DARPA, the agency that develops innovations for the Defense department and has been credited with hatching world-changing technologies such as ARPANET, which became the internet.
Time will tell if ARPA-H will lead to similar achievements in the realm of health. That’s what President Biden and Congress expect in return for funding ARPA-H at 2.5 billion dollars over three years.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
How will the agency figure out which projects to take on, especially with so many patient advocates for different diseases demanding moonshot funding for rapid progress?
I talked with Dr. Wegrzyn about the opportunities and challenges, what lessons ARPA-H is borrowing from Operation Warp Speed, how she decided on the first ARPA-H project that was announced recently, why a separate agency was needed instead of reforming HHS and the National Institutes of Health to be better at innovation, and how ARPA-H will make progress on disease prevention in addition to treatments for cancer, Alzheimer’s and diabetes, among many other health priorities.
Dr. Wegrzyn’s resume leaves no doubt of her suitability for this role. She was a program manager at DARPA where she focused on applying gene editing and synthetic biology to the goal of improving biosecurity. For her work there, she received the Superior Public Service Medal and, in case that wasn’t enough ARPA experience, she also worked at another ARPA that leads advanced projects in intelligence, called I-ARPA. Before that, she ran technical teams in the private sector working on gene therapies and disease diagnostics, among other areas. She has been a vice president of business development at Gingko Bioworks and headed innovation at Concentric by Gingko. Her training and education includes a PhD and undergraduate degree in applied biology from the Georgia Institute of Technology and she did her postdoc as an Alexander von Humboldt Fellow in Heidelberg, Germany.
Dr. Wegrzyn told me that she’s “in the hot seat.” The pressure is on for ARPA-H especially after the need and potential for health innovation was spot lit by the pandemic and the unprecedented speed of vaccine development. We'll soon find out if ARPA-H can produce gamechangers in health that are equivalent to DARPA’s creation of the internet.
Show links:
ARPA-H - https://arpa-h.gov/
Dr. Wegrzyn profile - https://arpa-h.gov/people/renee-wegrzyn/
Dr. Wegrzyn Twitter - https://twitter.com/rwegrzyn?lang=en
President Biden Announces Dr. Wegrzyn's appointment - https://www.whitehouse.gov/briefing-room/statement...
Leaps.org coverage of ARPA-H - https://leaps.org/arpa/
ARPA-H program for joints to heal themselves - https://arpa-h.gov/news/nitro/ -
ARPA-H virtual talent search - https://arpa-h.gov/news/aco-talent-search/

Dr. Renee Wegrzyn was appointed director of ARPA-H last October.
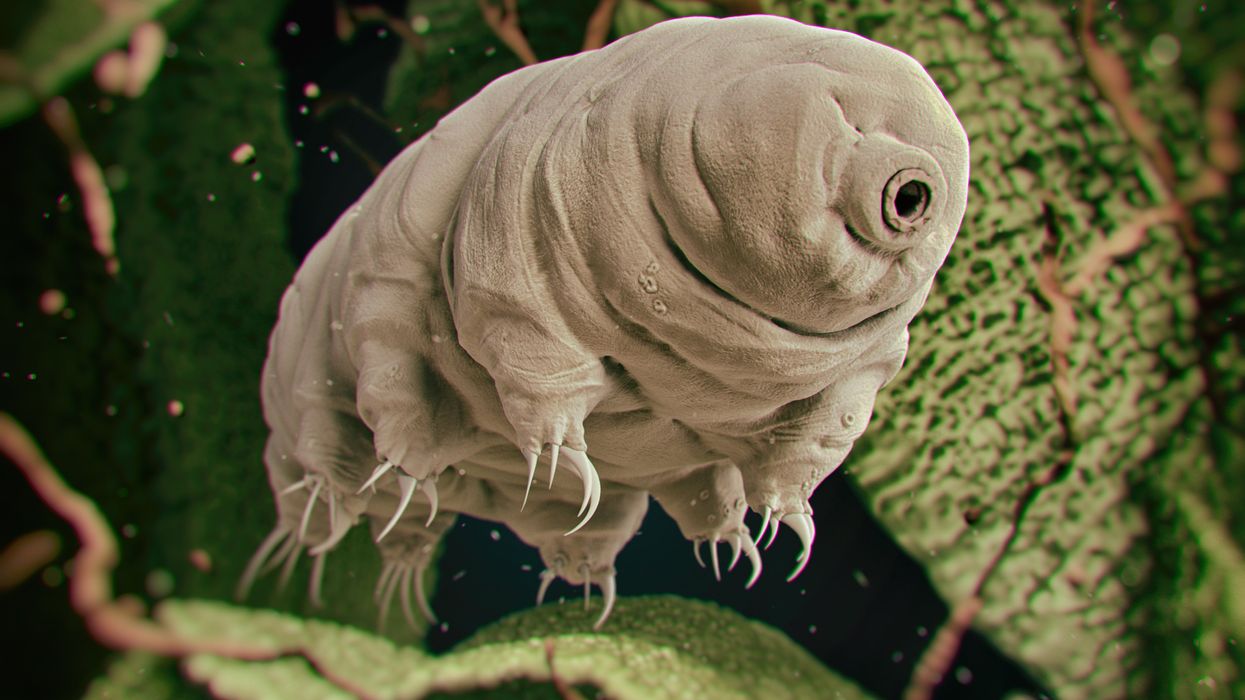
Tiny, tough “water bears” may help bring new vaccines and medicines to sub-Saharan Africa
Tardigrades can completely dehydrate and later rehydrate themselves, a survival trick that scientists are harnessing to preserve medicines in hot temperatures.
Microscopic tardigrades, widely considered to be some of the toughest animals on earth, can survive for decades without oxygen or water and are thought to have lived through a crash-landing on the moon. Also known as water bears, they survive by fully dehydrating and later rehydrating themselves – a feat only a few animals can accomplish. Now scientists are harnessing tardigrades’ talents to make medicines that can be dried and stored at ambient temperatures and later rehydrated for use—instead of being kept refrigerated or frozen.
Many biologics—pharmaceutical products made by using living cells or synthesized from biological sources—require refrigeration, which isn’t always available in many remote locales or places with unreliable electricity. These products include mRNA and other vaccines, monoclonal antibodies and immuno-therapies for cancer, rheumatoid arthritis and other conditions. Cooling is also needed for medicines for blood clotting disorders like hemophilia and for trauma patients.
Formulating biologics to withstand drying and hot temperatures has been the holy grail for pharmaceutical researchers for decades. It’s a hard feat to manage. “Biologic pharmaceuticals are highly efficacious, but many are inherently unstable,” says Thomas Boothby, assistant professor of molecular biology at University of Wyoming. Therefore, during storage and shipping, they must be refrigerated at 2 to 8 degrees Celsius (35 to 46 degrees Fahrenheit). Some must be frozen, typically at -20 degrees Celsius, but sometimes as low -90 degrees Celsius as was the case with the Pfizer Covid vaccine.
For Covid, fewer than 73 percent of the global population received even one dose. The need for refrigerated or frozen handling was partially to blame.
The costly cold chain
The logistics network that ensures those temperature requirements are met from production to administration is called the cold chain. This cold chain network is often unreliable or entirely lacking in remote, rural areas in developing nations that have malfunctioning electrical grids. “Almost all routine vaccines require a cold chain,” says Christopher Fox, senior vice president of formulations at the Access to Advanced Health Institute. But when the power goes out, so does refrigeration, putting refrigerated or frozen medical products at risk. Consequently, the mRNA vaccines developed for Covid-19 and other conditions, as well as more traditional vaccines for cholera, tetanus and other diseases, often can’t be delivered to the most remote parts of the world.
To understand the scope of the challenge, consider this: In the U.S., more than 984 million doses of Covid-19 vaccine have been distributed so far. Each one needed refrigeration that, even in the U.S., proved challenging. Now extrapolate to all vaccines and the entire world. For Covid, fewer than 73 percent of the global population received even one dose. The need for refrigerated or frozen handling was partially to blame.
Globally, the cold chain packaging market is valued at over $15 billion and is expected to exceed $60 billion by 2033.
Adobe Stock
Freeze-drying, also called lyophilization, which is common for many vaccines, isn’t always an option. Many freeze-dried vaccines still need refrigeration, and even medicines approved for storage at ambient temperatures break down in the heat of sub-Saharan Africa. “Even in a freeze-dried state, biologics often will undergo partial rehydration and dehydration, which can be extremely damaging,” Boothby explains.
The cold chain is also very expensive to maintain. The global pharmaceutical cold chain packaging market is valued at more than $15 billion, and is expected to exceed $60 billion by 2033, according to a report by Future Market Insights. This cost is only expected to grow. According to the consulting company Accenture, the number of medicines that require the cold chain are expected to grow by 48 percent, compared to only 21 percent for non-cold-chain therapies.
Tardigrades to the rescue
Tardigrades are only about a millimeter long – with four legs and claws, and they lumber around like bears, thus their nickname – but could provide a big solution. “Tardigrades are unique in the animal kingdom, in that they’re able to survive a vast array of environmental insults,” says Boothby, the Wyoming professor. “They can be dried out, frozen, heated past the boiling point of water and irradiated at levels that are thousands of times more than you or I could survive.” So, his team is gradually unlocking tardigrades’ survival secrets and applying them to biologic pharmaceuticals to make them withstand both extreme heat and desiccation without losing efficacy.
Boothby’s team is focusing on blood clotting factor VIII, which, as the name implies, causes blood to clot. Currently, Boothby is concentrating on the so-called cytoplasmic abundant heat soluble (CAHS) protein family, which is found only in tardigrades, protecting them when they dry out. “We showed we can desiccate a biologic (blood clotting factor VIII, a key clotting component) in the presence of tardigrade proteins,” he says—without losing any of its effectiveness.
The researchers mixed the tardigrade protein with the blood clotting factor and then dried and rehydrated that substance six times without damaging the latter. This suggests that biologics protected with tardigrade proteins can withstand real-world fluctuations in humidity.
Furthermore, Boothby’s team found that when the blood clotting factor was dried and stabilized with tardigrade proteins, it retained its efficacy at temperatures as high as 95 degrees Celsius. That’s over 200 degrees Fahrenheit, much hotter than the 58 degrees Celsius that the World Meteorological Organization lists as the hottest recorded air temperature on earth. In contrast, without the protein, the blood clotting factor degraded significantly. The team published their findings in the journal Nature in March.
Although tardigrades rarely live more than 2.5 years, they have survived in a desiccated state for up to two decades, according to Animal Diversity Web. This suggests that tardigrades’ CAHS protein can protect biologic pharmaceuticals nearly indefinitely without refrigeration or freezing, which makes it significantly easier to deliver them in locations where refrigeration is unreliable or doesn’t exist.
The tricks of the tardigrades
Besides the CAHS proteins, tardigrades rely on a type of sugar called trehalose and some other protectants. So, rather than drying up, their cells solidify into rigid, glass-like structures. As that happens, viscosity between cells increases, thereby slowing their biological functions so much that they all but stop.
Now Boothby is combining CAHS D, one of the proteins in the CAHS family, with trehalose. He found that CAHS D and trehalose each protected proteins through repeated drying and rehydrating cycles. They also work synergistically, which means that together they might stabilize biologics under a variety of dry storage conditions.
“We’re finding the protective effect is not just additive but actually is synergistic,” he says. “We’re keen to see if something like that also holds true with different protein combinations.” If so, combinations could possibly protect against a variety of conditions.
Commercialization outlook
Before any stabilization technology for biologics can be commercialized, it first must be approved by the appropriate regulators. In the U.S., that’s the U.S. Food and Drug Administration. Developing a new formulation would require clinical testing and vast numbers of participants. So existing vaccines and biologics likely won’t be re-formulated for dry storage. “Many were developed decades ago,” says Fox. “They‘re not going to be reformulated into thermo-stable vaccines overnight,” if ever, he predicts.
Extending stability outside the cold chain, even for a few days, can have profound health, environmental and economic benefits.
Instead, this technology is most likely to be used for the new products and formulations that are just being created. New and improved vaccines will be the first to benefit. Good candidates include the plethora of mRNA vaccines, as well as biologic pharmaceuticals for neglected diseases that affect parts of the world where reliable cold chain is difficult to maintain, Boothby says. Some examples include new, more effective vaccines for malaria and for pathogenic Escherichia coli, which causes diarrhea.
Tallying up the benefits
Extending stability outside the cold chain, even for a few days, can have profound health, environmental and economic benefits. For instance, MenAfriVac, a meningitis vaccine (without tardigrade proteins) developed for sub-Saharan Africa, can be stored at up to 40 degrees Celsius for four days before administration. “If you have a few days where you don’t need to maintain the cold chain, it’s easier to transport vaccines to remote areas,” Fox says, where refrigeration does not exist or is not reliable.
Better health is an obvious benefit. MenAfriVac reduced suspected meningitis cases by 57 percent in the overall population and more than 99 percent among vaccinated individuals.
Lower healthcare costs are another benefit. One study done in Togo found that the cold chain-related costs increased the per dose vaccine price up to 11-fold. The ability to ship the vaccines using the usual cold chain, but transporting them at ambient temperatures for the final few days cut the cost in half.
There are environmental benefits, too, such as reducing fuel consumption and greenhouse gas emissions. Cold chain transports consume 20 percent more fuel than non-cold chain shipping, due to refrigeration equipment, according to the International Trade Administration.
A study by researchers at Johns Hopkins University compared the greenhouse gas emissions of the new, oral Vaxart COVID-19 vaccine (which doesn’t require refrigeration) with four intramuscular vaccines (which require refrigeration or freezing). While the Vaxart vaccine is still in clinical trials, the study found that “up to 82.25 million kilograms of CO2 could be averted by using oral vaccines in the U.S. alone.” That is akin to taking 17,700 vehicles out of service for one year.
Although tardigrades’ protective proteins won’t be a component of biologic pharmaceutics for several years, scientists are proving that this approach is viable. They are hopeful that a day will come when vaccines and biologics can be delivered anywhere in the world without needing refrigerators or freezers en route.