Smartwatches can track COVID-19 symptoms, study finds

University of Michigan researchers found that new signals embedded in heart rate indicated when individuals were infected with COVID-19 and how ill they became.
If a COVID-19 infection develops, a wearable device may eventually be able to clue you in. A study at the University of Michigan found that a smartwatch can monitor how symptoms progress.
The study evaluated the effects of COVID-19 with various factors derived from heart-rate data. This method also could be employed to detect other diseases, such as influenza and the common cold, at home or when medical resources are limited, such as during a pandemic or in developing countries.
Tracking students and medical interns across the country, the University of Michigan researchers found that new signals embedded in heart rate indicated when individuals were infected with COVID-19 and how ill they became.
For instance, they discovered that individuals with COVID-19 experienced an increase in heart rate per step after the onset of their symptoms. Meanwhile, people who reported a cough as one of their COVID-19 symptoms had a much more elevated heart rate per step than those without a cough.
“We previously developed a variety of algorithms to analyze data from wearable devices. So, when the COVID-19 pandemic hit, it was only natural to apply some of these algorithms to see if we can get a better understanding of disease progression,” says Caleb Mayer, a doctoral student in mathematics at the University of Michigan and a co-first author of the study.
People may not internally sense COVID-19’s direct impact on the heart, but “heart rate is a vital sign that gives a picture of overall health," says Daniel Forger, a University of Michigan professor.
Millions of people are tracking their heart rate through wearable devices. This information is already generating a tremendous amount of data for researchers to analyze, says co-author Daniel Forger, professor of mathematics and research professor of computational medicine and bioinformatics at the University of Michigan.
“Heart rate is affected by many different physiological signals,” Forger explains. “For instance, if your lungs aren’t functioning properly, your heart may need to beat faster to meet metabolic demands. Your heart rate has a natural daily rhythm governed by internal biological clocks.” While people may not internally sense COVID-19’s direct impact on the heart, he adds that “heart rate is a vital sign that gives a picture of overall health.”
Among the total of 2,164 participants who enrolled in the student study, 72 undergraduate and graduate students contracted COVID-19, providing wearable data from 50 days before symptom onset to 14 days after. The researchers also analyzed this type of data for 43 medical interns from the Intern Health Study by the Michigan Neuroscience Institute and 29 individuals (who are not affiliated with the university) from the publicly available dataset.
Participants could wear the device on either wrist. They also documented their COVID-19 symptoms, such as fever, shortness of breath, cough, runny nose, vomiting, diarrhea, body aches, loss of taste, loss of smell, and sore throat.
Experts not involved in the study found the research to be productive. “This work is pioneering and reveals exciting new insights into the many important ways that we can derive clinically significant information about disease progression from consumer-grade wearable devices,” says Lisa A. Marsch, director of the Center for Technology and Behavioral Health and a professor in the Geisel School of Medicine at Dartmouth College. “Heart-rate data are among the highest-quality data that can be obtained via wearables.”
Beyond the heart, she adds, “Wearable devices are providing novel insights into individuals’ physiology and behavior in many health domains.” In particular, “this study beautifully illustrates how digital-health methodologies can markedly enhance our understanding of differences in individuals’ experience with disease and health.”
Previous studies had demonstrated that COVID-19 affects cardiovascular functions. Capitalizing on this knowledge, the University of Michigan endeavor took “a giant step forward,” says Gisele Oda, a researcher at the Institute of Biosciences at the University of Sao Paulo in Brazil and an expert in chronobiology—the science of biological rhythms. She commends the researchers for developing a complex algorithm that “could extract useful information beyond the established knowledge that heart rate increases and becomes more irregular in COVID patients.”
Wearable devices open the possibility of obtaining large-scale, long, continuous, and real-time heart-rate data on people performing everyday activities or while sleeping. “Importantly, the conceptual basis of this algorithm put circadian rhythms at the center stage,” Oda says, referring to the physical, mental, and behavioral changes that follow a 24-hour cycle. “What we knew before was often based on short-time heart rate measured at any time of day,” she adds, while noting that heart rate varies between day and night and also changes with activity.
However, without comparison to a control group of people having the common flu, it is difficult to determine if the heart-rate signals are unique to COVID-19 or also occur with other illnesses, says John Torous, an assistant professor of psychiatry at Harvard Medical School who has researched wearable devices. In addition, he points to recent data showing that many wearables, which work by beaming light through the skin, may be less accurate in people with darker skin due to variations in light absorption.
While the results sound interesting, they lack the level of conclusive evidence that would be needed to transform how physicians care for patients. “But it is a good step in learning more about what these wearables can tell us,” says Torous, who is also director of digital psychiatry at Beth Israel Deaconess Medical Center, a Harvard affiliate, in Boston. A follow-up step would entail replicating the results in a different pool of people to “help us realize the full value of this work.”
It is important to note that this research was conducted in university settings during the early phases of the pandemic, with remote learning in full swing amid strict isolation and quarantine mandates in effect. The findings demonstrate that physiological monitoring can be performed using consumer-grade wearable sensors, allowing research to continue without in-person contact, says Sung Won Choi, a professor of pediatrics at the University of Michigan who is principal investigator of the student study.
“The worldwide COVID-19 pandemic interrupted a lot of activities that relied on face-to-face interactions, including clinical research,” Choi says. “Mobile technology proved to be tremendously beneficial during that time, because it allowed us to collect detailed physiological data from research participants remotely over an entire semester.” In fact, the researchers did not have any in-person contact with the students involved in the study. “Everything was done virtually," Choi explains. "Importantly, their willingness to participate in research and share data during this historical time, combined with the capacity of secure cloud storage and novel mathematical analytics, enabled our research teams to identify unique patterns in heart-rate data associated with COVID-19.”
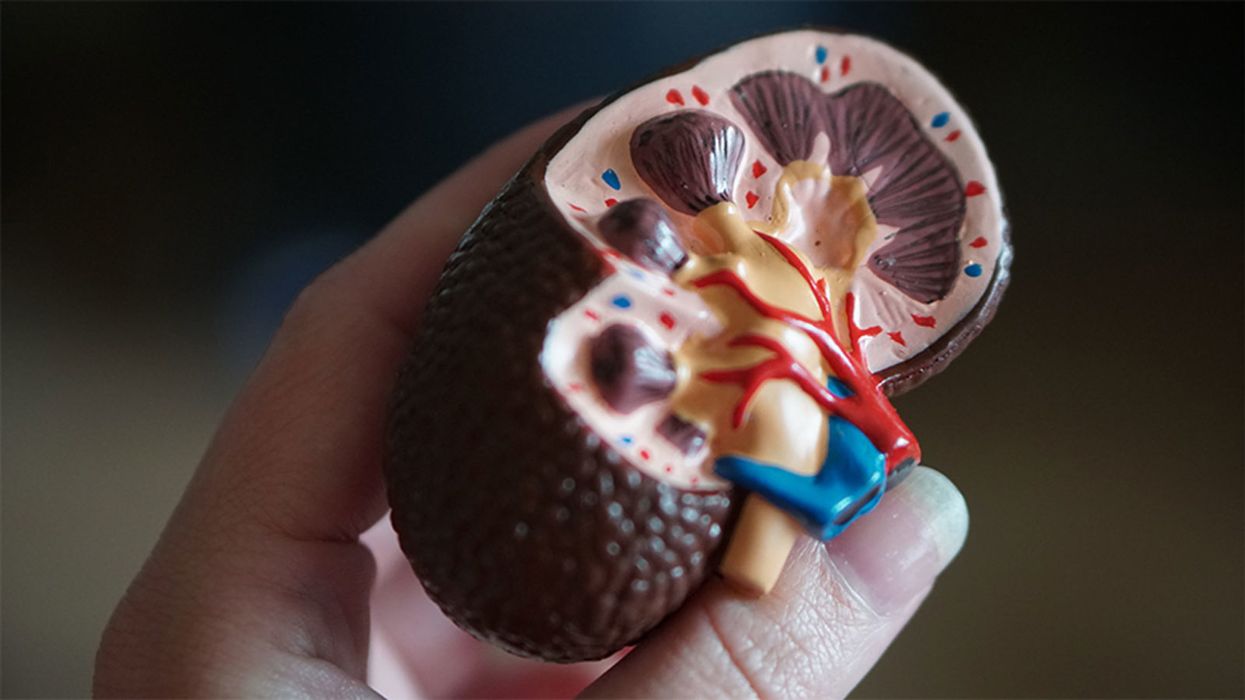
A model of a human kidney.
Science's dream of creating perfect custom organs on demand as soon as a patient needs one is still a long way off. But tiny versions are already serving as useful research tools and stepping stones toward full-fledged replacements.
Although organoids cannot yet replace kidneys, they are invaluable tools for research.
The Lowdown
Australian researchers have grown hundreds of mini human kidneys in the past few years. Known as organoids, they function much like their full-grown counterparts, minus a few features due to a lack of blood supply.
Cultivated in a petri dish, these kidneys are still a shadow of their human counterparts. They grow no larger than one-sixth of an inch in diameter; fully developed organs are up to five inches in length. They contain no more than a few dozen nephrons, the kidney's individual blood-filtering unit, whereas a fully-grown kidney has about 1 million nephrons. And the dish variety live for just a few weeks.
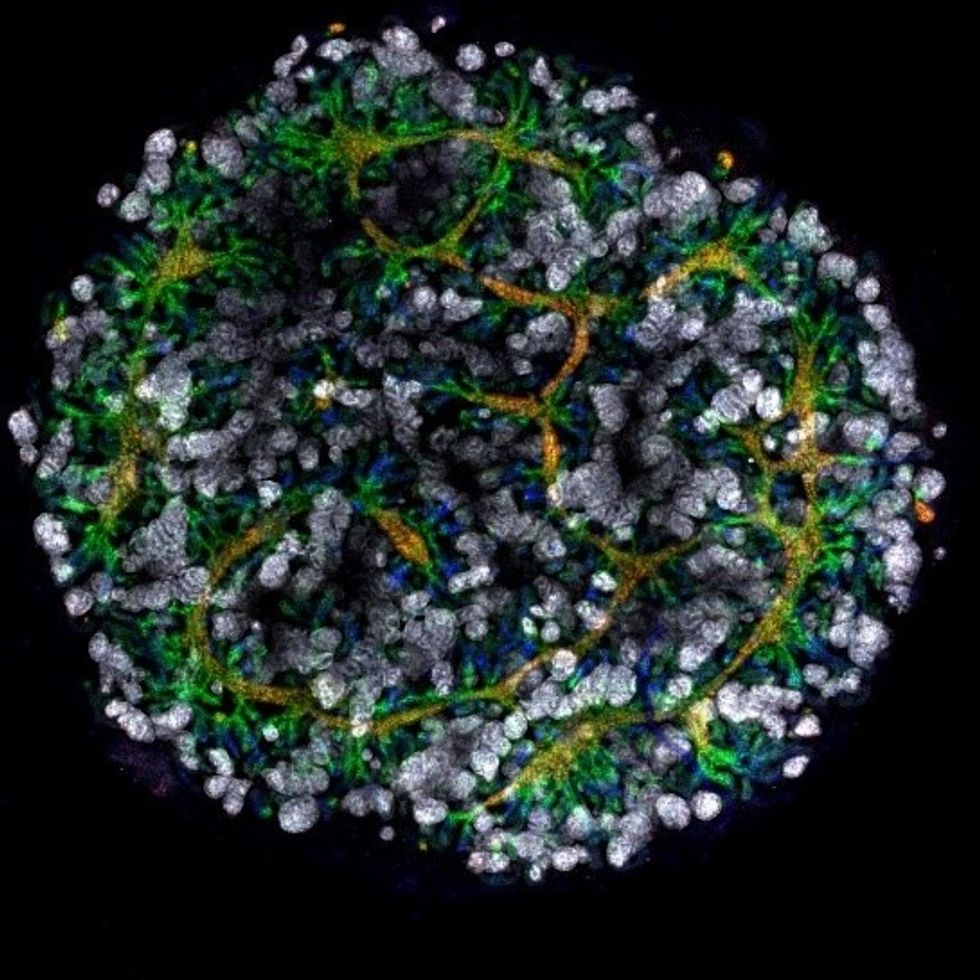
An organoid kidney created by the Murdoch Children's Institute in Melbourne, Australia.
Photo Credit: Shahnaz Khan.
But Melissa Little, head of the kidney research laboratory at the Murdoch Children's Institute in Melbourne, says these organoids are invaluable tools for research. Although renal failure is rare in children, more than half of those who suffer from such a disorder inherited it.
The mini kidneys enable scientists to better understand the progression of such disorders because they can be grown with a patient's specific genetic condition.
Mature stem cells can be extracted from a patient's blood sample and then reprogrammed to become like embryonic cells, able to turn into any type of cell in the body. It's akin to walking back the clock so that the cells regain unlimited potential for development. (The Japanese scientist who pioneered this technique was awarded the Nobel Prize in 2012.) These "induced pluripotent stem cells" can then be chemically coaxed to grow into mini kidneys that have the patient's genetic disorder.
"The (genetic) defects are quite clear in the organoids, and they can be monitored in the dish," Little says. To date, her research team has created organoids from 20 different stem cell lines.
Medication regimens can also be tested on the organoids, allowing specific tailoring for each patient. For now, such testing remains restricted to mice, but Little says it eventually will be done on human organoids so that the results can more accurately reflect how a given patient will respond to particular drugs.
Next Steps
Although these organoids cannot yet replace kidneys, Little says they may plug a huge gap in renal care by assisting in developing new treatments for chronic conditions. Currently, most patients with a serious kidney disorder see their options narrow to dialysis or organ transplantation. The former not only requires multiple sessions a week, but takes a huge toll on patient health.
Ten percent of older patients on dialysis die every year in the U.S. Aside from the physical trauma of organ transplantation, finding a suitable donor outside of a family member can be difficult.
"This is just another great example of the potential of pluripotent stem cells."
Meanwhile, the ongoing creation of organoids is supplying Little and her colleagues with enough information to create larger and more functional organs in the future. According to Little, researchers in the Netherlands, for example, have found that implanting organoids in mice leads to the creation of vascular growth, a potential pathway toward creating bigger and better kidneys.
And while Little acknowledges that creating a fully-formed custom organ is the ultimate goal, the mini organs are an important bridge step.
"This is just another great example of the potential of pluripotent stem cells, and I am just passionate to see it do some good."
Phil Gutis, an Alzheimer's patient who participated in a failed clinical trial, poses with his dog Abe.
Phil Gutis never had a stellar memory, but when he reached his early 50s, it became a problem he could no longer ignore. He had trouble calculating how much to tip after a meal, finding things he had just put on his desk, and understanding simple driving directions.
From 1998-2017, industry sources reported 146 failed attempts at developing Alzheimer's drugs.
So three years ago, at age 54, he answered an ad for a drug trial seeking people experiencing memory issues. He scored so low in the memory testing he was told something was wrong. M.R.I.s and PET scans confirmed that he had early-onset Alzheimer's disease.
Gutis, who is a former New York Times reporter and American Civil Liberties Union spokesman, felt fortunate to get into an advanced clinical trial of a new treatment for Alzheimer's disease. The drug, called aducanumab, had shown promising results in earlier studies.
Four years of data had found that the drug effectively reduced the burden of protein fragments called beta-amyloids, which destroy connections between nerve cells. Amyloid plaques are found in the brains of patients with Alzheimer's disease and are associated with impairments in thinking and memory.
Gutis eagerly participated in the clinical trial and received 35 monthly infusions. "For the first 20 infusions, I did not know whether I was receiving the drug or the placebo," he says. "During the last 15 months, I received aducanumab. But it really didn't matter if I was receiving the drug or the placebo because on March 21, the trial was stopped because [the drug company] Biogen found that the treatments were ineffective."
The news was devastating to the trial participants, but also to the Alzheimer's research community. Earlier this year, another pharmaceutical company, Roche, announced it was discontinuing two of its Alzheimer's clinical trials. From 1998-2017, industry sources reported 146 failed attempts at developing Alzheimer's drugs. There are five prescription drugs approved to treat its symptoms, but a cure remains elusive. The latest failures have left researchers scratching their heads about how to approach attacking the disease.
The failure of aducanumab was also another setback for the estimated 5.8 million people who have Alzheimer's in the United States. Of these, around 5.6 million are older than 65 and 200,000 suffer from the younger-onset form, including Gutis.
Gutis is understandably distraught about the cancellation of the trial. "I really had hopes it would work. So did all the patients."
While drug companies have failed so far, another group is stepping up to expedite the development of a cure: venture philanthropists.
For now, he is exercising every day to keep his blood flowing, which is supposed to delay the progression of the disease, and trying to eat a low-fat diet. "But I know that none of it will make a difference. Alzheimer's is a progressive disease. There are no treatments to delay it, let alone cure it."
But while drug companies have failed so far, another group is stepping up to expedite the development of a cure: venture philanthropists. These are successful titans of industry and dedicated foundations who are donating large sums of money to fill a much-needed void – funding research to look for new biomarkers.
Biomarkers are neurochemical indicators that can be used to detect the presence of a disease and objectively measure its progression. There are currently no validated biomarkers for Alzheimer's, but researchers are actively studying promising candidates. The hope is that they will find a reliable way to identify the disease even before the symptoms of mental decline show up, so that treatments can be directed at a very early stage.
Howard Fillit, Founding Executive Director and Chief Science Officer of the Alzheimer's Drug Discovery Foundation, says, "We need novel biomarkers to diagnose Alzheimer's disease and related dementias. But pharmaceutical companies don't put money into biomarkers research."
One of the venture philanthropists who has recently stepped up to the task is Bill Gates. In January 2018, he announced his father had Alzheimer's disease in an interview on the Today Show with Maria Shriver, whose father Sargent Shriver, died of Alzheimer's disease in 2011. Gates told Ms. Shriver that he had invested $100 million into Alzheimer's research, with $50 million of his donation going to Dementia Discovery Fund, which looks for new cures and treatments.
That August, Gates joined other investors in a new fund called Diagnostics Accelerator. The project aims to supports researchers looking to speed up new ideas for earlier and better diagnosis of the disease.
Gates and other donors committed more than $35 million to help launch it, and this April, Jeff and Mackenzie Bezos joined the coalition, bringing the current program funding to nearly $50 million.
"It makes sense that a challenge this significant would draw the attention of some of the world's leading thinkers."
None of these funders stand to make a profit on their donation, unlike traditional research investments by drug companies. The standard alternatives to such funding have upsides -- and downsides.
As Bill Gates wrote on his blog, "Investments from governments or charitable organizations are fantastic at generating new ideas and cutting-edge research -- but they're not always great at creating usable products, since no one stands to make a profit at the end of the day.
"Venture capital, on the other end of the spectrum, is more likely to develop a test that will reach patients, but its financial model favors projects that will earn big returns for investors. Venture philanthropy splits the difference. It incentivizes a bold, risk-taking approach to research with an end goal of a real product for real patients. If any of the projects backed by Diagnostics Accelerator succeed, our share of the financial windfall goes right back into the fund."
Gutis said he is thankful for any attention given to finding a cure for Alzheimer's.
"Most doctors and scientists will tell you that we're still in the dark ages when it comes to fully understanding how the brain works, let alone figuring out the cause or treatment for Alzheimer's.
"It makes sense that a challenge this significant would draw the attention of some of the world's leading thinkers. I only hope they can be more successful with their entrepreneurial approach to finding a cure than the drug companies have been with their more traditional paths."