New therapy may improve stem cell transplants for blood cancers

Ivan Dimov, Jeroen Bekaert and Nate Fernhoff - pictured here - recognized the need for a more effective cell sorting technology to reduce the risk of Graft vs Host disease, which affects many cancer patients after receiving stem cell transplants.
In 2018, Robyn was diagnosed with myelofibrosis, a blood cancer causing chronic inflammation and scarring. As a research scientist by training, she knew she had limited options. A stem cell transplant is a terminally ill patient's best chance for survival against blood cancers, including leukaemia. It works by destroying a patient's cancer cells and replacing them with healthy cells from a donor.
However, there is a huge risk of Graft vs Host disease (GVHD), which affects around 30-40% of recipients. Patients receive billions of cells in a stem cell transplant but only a fraction are beneficial. The rest can attack healthy tissue leading to GVHD. It affects the skin, gut and lungs and can be truly debilitating.
Currently, steroids are used to try and prevent GVHD, but they have many side effects and are effective in only 50% of cases. “I spoke with my doctors and reached out to patients managing GVHD,” says Robyn, who prefers not to use her last name for privacy reasons. “My concerns really escalated for what I might face post-transplant.”
Then she heard about a new highly precise cell therapy developed by a company called Orca Bio, which gives patients more beneficial cells and fewer cells that cause GVHD. She decided to take part in their phase 2 trial.
How It Works
In stem cell transplants, patients receive immune cells and stem cells. The donor immune cells or T cells attack and kill malignant cells. This is the graft vs leukaemia effect (GVL). The stem cells generate new healthy cells.
Unfortunately, T cells can also cause GVHD, but a rare subset of T cells, called T regulatory cells, can actually prevent GVHD.
Orca’s cell sorting technology distinguishes T regulatory cells from stem cells and conventional T cells on a large scale. It’s this cell sorting technology which has enabled them to create their new cell therapy, called Orca T. It contains a precise combination of stem cells and immune cells with more T regulatory cells and fewer conventional T cells than in a typical stem cell transplant.
“Ivan Dimov’s idea was to spread out the cells, keep them stationary and then use laser scanning to sort the cells,” explains Nate Fernhoff, co-founder of Orca Bio. “The beauty here is that lasers don't care how quickly you move them.”
Over the past 40 years, scientists have been trying to create stem cell grafts that contain the beneficial cells whilst removing the cells that cause GVHD. What makes it even harder is that most transplant centers aren’t able to manipulate grafts to create a precise combination of cells.
Innovative Cell Sorting
Ivan Dimov, Jeroen Bekaert and Nate Fernhoff came up with the idea behind Orca as postdocs at Stanford, working with cell pioneer Irving Weissman. They recognised the need for a more effective cell sorting technology. In a small study at Stanford, Professor Robert Negrin had discovered a combination of T cells, T regulatory cells and stem cells which prevented GVHD but retained the beneficial graft vs leukaemia effect (GVL). However, manufacturing was problematic. Conventional cell sorting is extremely slow and specific. Negrin was only able to make seven highly precise products, for seven patients, in a year. Annual worldwide cases of blood cancer number over 1.2 million.
“We started Orca with this idea: how do we use manufacturing solutions to impact cell therapies,” co-founder Fernhoff reveals. In conventional cell sorting, cells move past a stationary laser which analyses each cell. But cells can only be moved so quickly. At a certain point they start to experience stress and break down. This makes it very difficult to sort the 100 billion cells from a donor in a stem cell transplant.
“Ivan Dimov’s idea was to spread out the cells, keep them stationary and then use laser scanning to sort the cells,” Fernhoff explains. “The beauty here is that lasers don't care how quickly you move them.” They developed this technology and called it Orca Sort. It enabled Orca to make up to six products per week in the first year of manufacturing.
Every product Orca makes is for one patient. The donor is uniquely matched to the patient. They have to carry out the cell sorting procedure each time. Everything also has to be done extremely quickly. They infuse fresh living cells from the donor's vein to the patient's within 72 hours.
“We’ve treated almost 200 patients in all the Orca trials, and you can't do that if you don't fix the manufacturing process,” Fernhoff says. “We're working on what we think is an incredibly promising drug, but it's all been enabled by figuring out how to make a high precision cell therapy at scale.”
Clinical Trials
Orca revealed the results of their phase 1b and phase 2 trials at the end of last year. In their phase 2 trial only 3% of the 29 patients treated with Orca T cell therapy developed chronic GVHD in the first year after treatment. Comparatively, 43% of the 95 patients given a conventional stem cell transplant in a contemporary Stanford trial developed chronic GVHD. Of the 109 patients tested in phase 1b and phase 2 trials, 74% using Orca T didn't relapse or develop any form of GVHD compared to 34% in the control trial.
“Until a randomised study is done, we can make no assumption about the relative efficacy of this approach," says Jeff Szer, professor of haematology at the Royal Melbourne Hospital. "But the holy grail of separating GVHD and GVL is still there and this is a step towards realising that dream.”
Stan Riddell, an immunology professor, at Fred Hutchinson Cancer Centre, believes Orca T is highly promising. “Orca has advanced cell selection processes with innovative methodology and can engineer grafts with greater precision to add cell subsets that may further contribute to beneficial outcomes,” he says. “Their results in phase 1 and phase 2 studies are very exciting and offer the potential of providing a new standard of care for stem cell transplant.”
However, though it is an “intriguing step,” there’s a need for further testing, according to Jeff Szer, a professor of haematology at the Peter MacCallum Cancer Centre at the Royal Melbourne Hospital.
“The numbers tested were tiny and comparing the outcomes to anything from a phase 1/2 setting is risky,” says Szer. “Until a randomised study is done, we can make no assumption about the relative efficacy of this approach. But the holy grail of separating GVHD and GVL is still there and this is a step towards realising that dream.”
The Future
The team is soon starting Phase 3 trials for Orca T. Its previous success has led them to develop Orca Q, a cell therapy for patients who can't find an exact donor match. Transplants for patients who are only a half-match or mismatched are not widely used because there is a greater risk of GVHD. Orca Q has the potential to control GVHD even more and improve access to transplants for many patients.
Fernhoff hopes they’ll be able to help people not just with blood cancers but also with other blood and immune disorders. If a patient has a debilitating disease which isn't life threatening, the risk of GVHD outweighs the potential benefits of a stem cell transplant. The Orca products could take away that risk.
Meanwhile, Robyn has no regrets about participating in the Phase 2 trial. “It was a serious decision to make but I'm forever grateful that I did,” she says. “I have resumed a quality of life aligned with how I felt pre-transplant. I have not had a single issue with GVHD.”
“I want to be able to get one of these products to every patient who could benefit from it,” Fernhoff says. “It's really exciting to think about how Orca's products could be applied to all sorts of autoimmune disorders.”
Researchers advance drugs that treat pain without addiction
New therapies are using creative approaches that target the body’s sensory neurons, which send pain signals to the brain.
Opioids are one of the most common ways to treat pain. They can be effective but are also highly addictive, an issue that has fueled the ongoing opioid crisis. In 2020, an estimated 2.3 million Americans were dependent on prescription opioids.
Opioids bind to receptors at the end of nerve cells in the brain and body to prevent pain signals. In the process, they trigger endorphins, so the brain constantly craves more. There is a huge risk of addiction in patients using opioids for chronic long-term pain. Even patients using the drugs for acute short-term pain can become dependent on them.
Scientists have been looking for non-addictive drugs to target pain for over 30 years, but their attempts have been largely ineffective. “We desperately need alternatives for pain management,” says Stephen E. Nadeau, a professor of neurology at the University of Florida.
A “dimmer switch” for pain
Paul Blum is a professor of biological sciences at the University of Nebraska. He and his team at Neurocarrus have created a drug called N-001 for acute short-term pain. N-001 is made up of specially engineered bacterial proteins that target the body’s sensory neurons, which send pain signals to the brain. The proteins in N-001 turn down pain signals, but they’re too large to cross the blood-brain barrier, so they don’t trigger the release of endorphins. There is no chance of addiction.
When sensory neurons detect pain, they become overactive and send pain signals to the brain. “We wanted a way to tone down sensory neurons but not turn them off completely,” Blum reveals. The proteins in N-001 act “like a dimmer switch, and that's key because pain is sensation overstimulated.”
Blum spent six years developing the drug. He finally managed to identify two proteins that form what’s called a C2C complex that changes the structure of a subunit of axons, the parts of neurons that transmit electrical signals of pain. Changing the structure reduces pain signaling.
“It will be a long path to get to a successful clinical trial in humans," says Stephen E. Nadeau, professor of neurology at the University of Florida. "But it presents a very novel approach to pain reduction.”
Blum is currently focusing on pain after knee and ankle surgery. Typically, patients are treated with anesthetics for a short time after surgery. But anesthetics usually only last for 4 to 6 hours, and long-term use is toxic. For some, the pain subsides. Others continue to suffer after the anesthetics have worn off and start taking opioids.
N-001 numbs sensation. It lasts for up to 7 days, much longer than any anesthetic. “Our goal is to prolong the time before patients have to start opioids,” Blum says. “The hope is that they can switch from an anesthetic to our drug and thereby decrease the likelihood they're going to take the opioid in the first place.”
Their latest animal trial showed promising results. In mice, N-001 reduced pain-like behaviour by 90 percent compared to the control group. One dose became effective in two hours and lasted a week. A high dose had pain-relieving effects similar to an opioid.
Professor Stephen P. Cohen, director of pain operations at John Hopkins, believes the Neurocarrus approach has potential but highlights the need to go beyond animal testing. “While I think it's promising, it's an uphill battle,” he says. “They have shown some efficacy comparable to opioids, but animal studies don't translate well to people.”
Nadeau, the University of Florida neurologist, agrees. “It will be a long path to get to a successful clinical trial in humans. But it presents a very novel approach to pain reduction.”
Blum is now awaiting approval for phase I clinical trials for acute pain. He also hopes to start testing the drug's effect on chronic pain.
Learning from people who feel no pain
Like Blum, a pharmaceutical company called Vertex is focusing on treating acute pain after surgery. But they’re doing this in a different way, by targeting a sodium channel that plays a critical role in transmitting pain signals.
In 2004, Stephen Waxman, a neurology professor at Yale, led a search for genetic pain anomalies and found that biologically related people who felt no pain despite fractures, burns and even childbirth had mutations in the Nav1.7 sodium channel. Further studies in other families who experienced no pain showed similar mutations in the Nav1.8 sodium channel.
Scientists set out to modify these channels. Many unsuccessful efforts followed, but Vertex has now developed VX-548, a medicine to inhibit Nav1.8. Typically, sodium ions flow through sodium channels to generate rapid changes in voltage which create electrical pulses. When pain is detected, these pulses in the Nav1.8 channel transmit pain signals. VX-548 uses small molecules to inhibit the channel from opening. This blocks the flow of sodium ions and the pain signal. Because Nav1.8 operates only in peripheral nerves, located outside the brain, VX-548 can relieve pain without any risk of addiction.
"Frankly we need drugs for chronic pain more than acute pain," says Waxman.
The team just finished phase II clinical trials for patients following abdominoplasty surgery and bunionectomy surgery.
After abdominoplasty surgery, 76 patients were treated with a high dose of VX-548. Researchers then measured its effectiveness in reducing pain over 48 hours, using the SPID48 scale, in which higher scores are desirable. The score for Vertex’s drug was 110.5 compared to 72.7 in the placebo group, whereas the score for patients taking an opioid was 85.2. The study involving bunionectomy surgery showed positive results as well.
Waxman, who has been at the forefront of studies into Nav1.7 and Nav1.8, believes that Vertex's results are promising, though he highlights the need for further clinical trials.
“Blocking Nav1.8 is an attractive target,” he says. “[Vertex is] studying pain that is relatively simple and uniform, and that's key to having a drug trial that is informative. But the study needs to be replicated and frankly we need drugs for chronic pain more than acute pain. If this is borne out by additional studies, it's one important step in a journey.”
Vertex will be launching phase III trials later this year.
Finding just the right amount of Nerve Growth Factor
Whereas Neurocarrus and Vertex are targeting short-term pain, a company called Levicept is concentrating on relieving chronic osteoarthritis pain. Around 32.5 million Americans suffer from osteoarthritis. Patients commonly take NSAIDs, or non-steroidal anti-inflammatory drugs, but they cannot be taken long-term. Some take opioids but they aren't very effective.
Levicept’s drug, Levi-04, is designed to modify a signaling pathway associated with pain. Nerve Growth Factor (NGF) is a neurotrophin: it’s involved in nerve growth and function. NGF signals by attaching to receptors. In pain there are excess neurotrophins attaching to receptors and activating pain signals.
“What Levi-04 does is it returns the natural equilibrium of neurotrophins,” says Simon Westbrook, the CEO and founder of Levicept. It stabilizes excess neurotrophins so that the NGF pathway does not signal pain. Levi-04 isn't addictive since it works within joints and in nerves outside the brain.
Westbrook was initially involved in creating an anti-NGF molecule for Pfizer called Tanezumab. At first, Tanezumab seemed effective in clinical trials and other companies even started developing their own versions. However, a problem emerged. Tanezumab caused rapidly progressive osteoarthritis, or RPOA, in some patients because it completely removed NGF from the system. NGF is not just involved in pain signalling, it’s also involved in bone growth and maintenance.
Levicept has found a way to modify the NGF pathway without completely removing NGF. They have now finished a small-scale phase I trial mainly designed to test safety rather than efficacy. “We demonstrated that Levi-04 is safe and that it bound to its target, NGF,” says Westbrook. It has not caused RPOA.
Professor Philip Conaghan, director of the Leeds Institute of Rheumatic and Musculoskeletal Medicine, believes that Levi-04 has potential but urges the need for caution. “At this early stage of development, their molecule looks promising for osteoarthritis pain,” he says. “They will have to watch out for RPOA which is a potential problem.”
Westbrook starts phase II trials with 500 patients this summer to check for potential side effects and test the drug’s efficacy.
There is a real push to find an effective alternative to opioids. “We have a lot of work to do,” says Professor Waxman. “But I am confident that we will be able to develop new, much more effective pain therapies.”
Tech-related injuries are becoming more common as many people depend on - and often develop addictions for - smart phones and computers.
In the 1990s, a mysterious virus spread throughout the Massachusetts Institute of Technology Artificial Intelligence Lab—or that’s what the scientists who worked there thought. More of them rubbed their aching forearms and massaged their cricked necks as new computers were introduced to the AI Lab on a floor-by-floor basis. They realized their musculoskeletal issues coincided with the arrival of these new computers—some of which were mounted high up on lab benches in awkward positions—and the hours spent typing on them.
Today, these injuries have become more common in a society awash with smart devices, sleek computers, and other gadgets. And we don’t just get hurt from typing on desktop computers; we’re massaging our sore wrists from hours of texting and Facetiming on phones, especially as they get bigger in size.
In 2007, the first iPhone measured 3.5-inches diagonally, a measurement known as the display size. That’s been nearly doubled by the newest iPhone 13 Pro, which has a 6.7-inch display. Other phones, too, like the Google Pixel 6 and the Samsung Galaxy S22, have bigger screens than their predecessors. Physical therapists and orthopedic surgeons have had to come up with names for a variety of new conditions: selfie elbow, tech neck, texting thumb. Orthopedic surgeon Sonya Sloan says she sees selfie elbow in younger kids and in women more often than men. She hears complaints related to technology once or twice a day.
The addictive quality of smartphones and social media means that people spend more time on their devices, which exacerbates injuries. According to Statista, 68 percent of those surveyed spent over three hours a day on their phone, and almost half spent five to six hours a day. Another report showed that people dedicate a third of their day to checking their phones, while the Media Effects Research Laboratory at Pennsylvania State University has found that bigger screens, ideal for entertainment purposes, immerse their users more than smaller screens. Oversized screens also provide easier navigation and more space for those with bigger hands or trouble seeing.
But others with conditions like arthritis can benefit from smaller phones. In March of 2016, Apple released the iPhone SE with a display size of 4.7 inches—an inch smaller than the iPhone 7, released that September. Apple has since come out with two more versions of the diminutive iPhone SE, one in 2020 and another in 2022.
These devices are now an inextricable part of our lives. So where does the burden of responsibility lie? Is it with consumers to adjust body positioning, get ergonomic workstations, and change habits to abate tech-related pain? Or should tech companies be held accountable?
Kavin Senapathy, a freelance science journalist, has the Google Pixel 6. She was drawn to the phone because Google marketed the Pixel 6’s camera as better at capturing different skin tones. But this phone boasts one of the largest display sizes on the market: 6.4 inches.
Senapathy was diagnosed with carpal and cubital tunnel syndromes in 2017 and fibromyalgia in 2019. She has had to create a curated ergonomic workplace setup, otherwise her wrists and hands get weak and tingly, and she’s had to adjust how she holds her phone to prevent pain flares.
Recently, Senapathy underwent an electromyography, or an EMG, in which doctors insert electrodes into muscles to measure their electrical activity. The electrical response of the muscles tells doctors whether the nerve cells and muscles are successfully communicating. Depending on her results, steroid shots and even surgery might be required. Senapathy wants to stick with her Pixel 6, but the pain she’s experiencing may push her to buy a smaller phone. Unfortunately, options for these modestly sized phones are more limited.
These devices are now an inextricable part of our lives. So where does the burden of responsibility lie? Is it with consumers like Senapathy to adjust body positioning, get ergonomic workstations, and change habits to abate tech-related pain? Or should tech companies be held accountable for creating addictive devices that lead to musculoskeletal injury?

Kavin Senapathy, a freelance journalist, bought the Google Pixel 6 because of its high-quality camera, but she’s had to adjust how she holds the oversized phone to prevent pain flares.
Kavin Senapathy
A one-size-fits-all mentality for smartphones will continue to lead to injuries because every user has different wants and needs. S. Shyam Sundar, the founder of Penn State’s lab on media effects and a communications professor, says the needs for mobility and portability conflict with the desire for greater visibility. “The best thing a company can do is offer different sizes,” he says.
Joanna Bryson, an AI ethics expert and professor at The Hertie School of Governance in Berlin, Germany, echoed these sentiments. “A lot of the lack of choice we see comes from the fact that the markets have consolidated so much,” she says. “We want to make sure there’s sufficient diversity [of products].”
Consumers can still maintain some control despite the ubiquity of tech. Sloan, the orthopedic surgeon, has to pester her son to change his body positioning when using his tablet. Our heads get heavier as they bend forward: at rest, they weigh 12 pounds, but bent 60 degrees, they weigh 60. “I have to tell him, ‘Raise your head, son!’” she says. It’s important, Sloan explains, to consider that growth and development will affect ligaments and bones in the neck, potentially making kids even more vulnerable to injuries from misusing gadgets. She recommends that parents limit their kids’ tech time to alleviate strain. She also suggested that tech companies implement a timer to remind us to change our body positioning.
In 2017, Nan-Wei Gong, a former contractor for Google, founded Figur8, which uses wearable trackers to measure muscle function and joint movement. It’s like physical therapy with biofeedback. “Each unique injury has a different biomarker,” says Gong. “With Figur8, you are comparing yourself to yourself.” This allows an individual to self-monitor for wear and tear and strengthen an injury in a way that’s efficient and designed for their body. Gong noticed that the work-from-home model during the COVID-19 pandemic created a new set of ergonomic problems that resulted in injuries. Figur8 provides real-time data for these injuries because “behavioral change requires feedback.”
Gong worked on a project called Jacquard while at Google. Textile experts weave conductive thread into their fabric, and the result is a patch of the fabric—like the cuff of a Levi’s jacket—that responds to commands on your smartphone. One swipe can call your partner or check the weather. It was designed with cyclists in mind who can’t easily check their phones, and it’s part of a growing movement in the tech industry to deliver creative, hands-free design. Gong thinks that engineers at large corporations like Google have accessibility in mind; it’s part of what drives their decisions for new products.
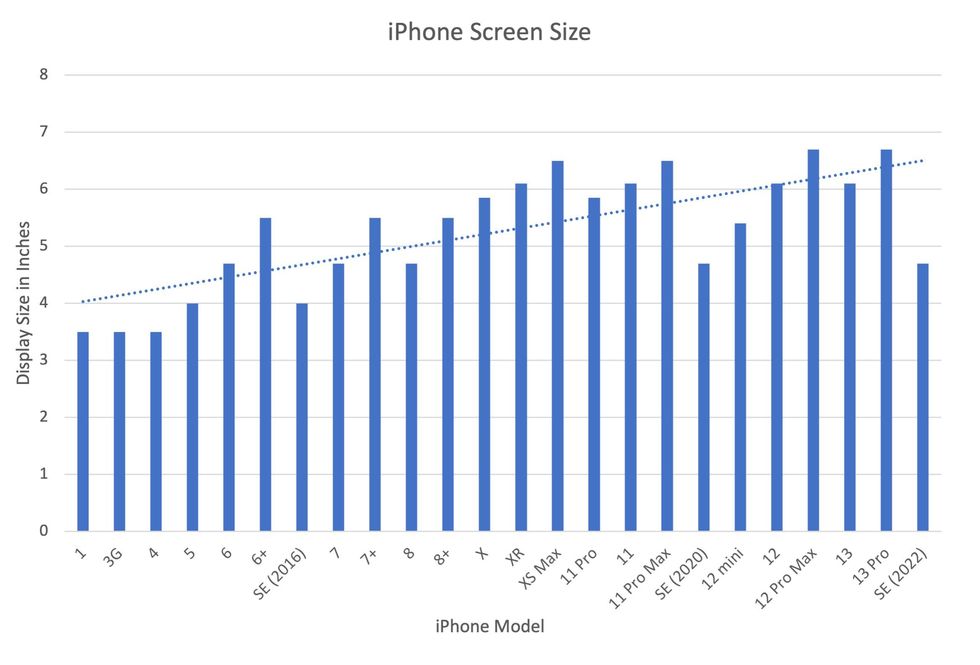
Display sizes of iPhones have become larger over time.
Sourced from Screenrant https://screenrant.com/iphone-apple-release-chronological-order-smartphone/ and Apple Tech Specs: https://www.apple.com/iphone-se/specs/
Back in Germany, Joanna Bryson reminds us that products like smartphones should adhere to best practices. These rules may be especially important for phones and other products with AI that are addictive. Disclosure, accountability, and regulation are important for AI, she says. “The correct balance will keep changing. But we have responsibilities and obligations to each other.” She was on an AI Ethics Council at Google, but the committee was disbanded after only one week due to issues with one of their members.
Bryson was upset about the Council’s dissolution but has faith that other regulatory bodies will prevail. OECD.AI, and international nonprofit, has drafted policies to regulate AI, which countries can sign and implement. “As of July 2021, 46 governments have adhered to the AI principles,” their website reads.
Sundar, the media effects professor, also directs Penn State’s Center for Socially Responsible AI. He says that inclusivity is a crucial aspect of social responsibility and how devices using AI are designed. “We have to go beyond first designing technologies and then making them accessible,” he says. “Instead, we should be considering the issues potentially faced by all different kinds of users before even designing them.”