New therapy may improve stem cell transplants for blood cancers

Ivan Dimov, Jeroen Bekaert and Nate Fernhoff - pictured here - recognized the need for a more effective cell sorting technology to reduce the risk of Graft vs Host disease, which affects many cancer patients after receiving stem cell transplants.
In 2018, Robyn was diagnosed with myelofibrosis, a blood cancer causing chronic inflammation and scarring. As a research scientist by training, she knew she had limited options. A stem cell transplant is a terminally ill patient's best chance for survival against blood cancers, including leukaemia. It works by destroying a patient's cancer cells and replacing them with healthy cells from a donor.
However, there is a huge risk of Graft vs Host disease (GVHD), which affects around 30-40% of recipients. Patients receive billions of cells in a stem cell transplant but only a fraction are beneficial. The rest can attack healthy tissue leading to GVHD. It affects the skin, gut and lungs and can be truly debilitating.
Currently, steroids are used to try and prevent GVHD, but they have many side effects and are effective in only 50% of cases. “I spoke with my doctors and reached out to patients managing GVHD,” says Robyn, who prefers not to use her last name for privacy reasons. “My concerns really escalated for what I might face post-transplant.”
Then she heard about a new highly precise cell therapy developed by a company called Orca Bio, which gives patients more beneficial cells and fewer cells that cause GVHD. She decided to take part in their phase 2 trial.
How It Works
In stem cell transplants, patients receive immune cells and stem cells. The donor immune cells or T cells attack and kill malignant cells. This is the graft vs leukaemia effect (GVL). The stem cells generate new healthy cells.
Unfortunately, T cells can also cause GVHD, but a rare subset of T cells, called T regulatory cells, can actually prevent GVHD.
Orca’s cell sorting technology distinguishes T regulatory cells from stem cells and conventional T cells on a large scale. It’s this cell sorting technology which has enabled them to create their new cell therapy, called Orca T. It contains a precise combination of stem cells and immune cells with more T regulatory cells and fewer conventional T cells than in a typical stem cell transplant.
“Ivan Dimov’s idea was to spread out the cells, keep them stationary and then use laser scanning to sort the cells,” explains Nate Fernhoff, co-founder of Orca Bio. “The beauty here is that lasers don't care how quickly you move them.”
Over the past 40 years, scientists have been trying to create stem cell grafts that contain the beneficial cells whilst removing the cells that cause GVHD. What makes it even harder is that most transplant centers aren’t able to manipulate grafts to create a precise combination of cells.
Innovative Cell Sorting
Ivan Dimov, Jeroen Bekaert and Nate Fernhoff came up with the idea behind Orca as postdocs at Stanford, working with cell pioneer Irving Weissman. They recognised the need for a more effective cell sorting technology. In a small study at Stanford, Professor Robert Negrin had discovered a combination of T cells, T regulatory cells and stem cells which prevented GVHD but retained the beneficial graft vs leukaemia effect (GVL). However, manufacturing was problematic. Conventional cell sorting is extremely slow and specific. Negrin was only able to make seven highly precise products, for seven patients, in a year. Annual worldwide cases of blood cancer number over 1.2 million.
“We started Orca with this idea: how do we use manufacturing solutions to impact cell therapies,” co-founder Fernhoff reveals. In conventional cell sorting, cells move past a stationary laser which analyses each cell. But cells can only be moved so quickly. At a certain point they start to experience stress and break down. This makes it very difficult to sort the 100 billion cells from a donor in a stem cell transplant.
“Ivan Dimov’s idea was to spread out the cells, keep them stationary and then use laser scanning to sort the cells,” Fernhoff explains. “The beauty here is that lasers don't care how quickly you move them.” They developed this technology and called it Orca Sort. It enabled Orca to make up to six products per week in the first year of manufacturing.
Every product Orca makes is for one patient. The donor is uniquely matched to the patient. They have to carry out the cell sorting procedure each time. Everything also has to be done extremely quickly. They infuse fresh living cells from the donor's vein to the patient's within 72 hours.
“We’ve treated almost 200 patients in all the Orca trials, and you can't do that if you don't fix the manufacturing process,” Fernhoff says. “We're working on what we think is an incredibly promising drug, but it's all been enabled by figuring out how to make a high precision cell therapy at scale.”
Clinical Trials
Orca revealed the results of their phase 1b and phase 2 trials at the end of last year. In their phase 2 trial only 3% of the 29 patients treated with Orca T cell therapy developed chronic GVHD in the first year after treatment. Comparatively, 43% of the 95 patients given a conventional stem cell transplant in a contemporary Stanford trial developed chronic GVHD. Of the 109 patients tested in phase 1b and phase 2 trials, 74% using Orca T didn't relapse or develop any form of GVHD compared to 34% in the control trial.
“Until a randomised study is done, we can make no assumption about the relative efficacy of this approach," says Jeff Szer, professor of haematology at the Royal Melbourne Hospital. "But the holy grail of separating GVHD and GVL is still there and this is a step towards realising that dream.”
Stan Riddell, an immunology professor, at Fred Hutchinson Cancer Centre, believes Orca T is highly promising. “Orca has advanced cell selection processes with innovative methodology and can engineer grafts with greater precision to add cell subsets that may further contribute to beneficial outcomes,” he says. “Their results in phase 1 and phase 2 studies are very exciting and offer the potential of providing a new standard of care for stem cell transplant.”
However, though it is an “intriguing step,” there’s a need for further testing, according to Jeff Szer, a professor of haematology at the Peter MacCallum Cancer Centre at the Royal Melbourne Hospital.
“The numbers tested were tiny and comparing the outcomes to anything from a phase 1/2 setting is risky,” says Szer. “Until a randomised study is done, we can make no assumption about the relative efficacy of this approach. But the holy grail of separating GVHD and GVL is still there and this is a step towards realising that dream.”
The Future
The team is soon starting Phase 3 trials for Orca T. Its previous success has led them to develop Orca Q, a cell therapy for patients who can't find an exact donor match. Transplants for patients who are only a half-match or mismatched are not widely used because there is a greater risk of GVHD. Orca Q has the potential to control GVHD even more and improve access to transplants for many patients.
Fernhoff hopes they’ll be able to help people not just with blood cancers but also with other blood and immune disorders. If a patient has a debilitating disease which isn't life threatening, the risk of GVHD outweighs the potential benefits of a stem cell transplant. The Orca products could take away that risk.
Meanwhile, Robyn has no regrets about participating in the Phase 2 trial. “It was a serious decision to make but I'm forever grateful that I did,” she says. “I have resumed a quality of life aligned with how I felt pre-transplant. I have not had a single issue with GVHD.”
“I want to be able to get one of these products to every patient who could benefit from it,” Fernhoff says. “It's really exciting to think about how Orca's products could be applied to all sorts of autoimmune disorders.”
Two students had an idea at a scrapyard. They went on to "upcycle" 80,000 airbags, 100,000 seatbelts and 28,000 belt buckles – the equivalent of 60 tons of car trash
Luckily, two college freshmen at the Rotterdam School of Management, Erasmus University, were naïve enough to take their bicycles to the scrapyard. In a previous stroke of fortune, the freshmen, Adrian Goosses and Michael Widmann, had been assigned as roommates and had quickly hit it off. Now they were looking for a cool recycling project for their first semester “strategic entrepreneurship” course—maybe they could turn old tires into comfortable lounge chairs, they thought.
“Everybody gets around by bike in Rotterdam,” says Goosses, now 32, from his home in Cologne, Germany. “The tires were way too heavy and cumbersome to transport by bike,” Widmann chimes in via Zoom from Bolzano, Italy, where he lives.
Sifting through the car trash for something handier led the two students to an idea that has since flourished: Could the airbag and seatbelts from a banged up compact car be salvaged and turned into a sustainable backpack? The size of the airbag was already a natural fit. The seatbelts made perfect shoulder straps. After returning from the scrapyard, “We stitched the prototype together by hand with a needle and yarn,” says Goosses. “Yet we didn’t even know how to sew!”
Much to their surprise, their classmates responded with so much enthusiasm to their “trash bag” concept that it convinced the two innovators to keep going. Every semester, they improved the prototype further. With the help of YouTube videos, they taught themselves how to sew. Because modern electric sewing machines had a difficult time breaking through the tough nylon of the airbags, Goosses and Widmann went to a second-hand shop and purchased an ancient Singer from 1880 for 10 Euros. They dyed the first airbags in a saucepan in the garden outside of the apartment they shared.
“By the time we graduated, we had a presentable prototype and a business plan,” Goosses says.
Despite their progress, Goosses and Widmann are up against a problem that’s immense: Cars are notoriously difficult to recycle because many parts are considered toxic waste.
It’s an example of “upcycling,” when you spot a potential new use in something that’s been trashed, shelved or otherwise retired. The approach has received increasing attention and support from the U.S. Environmental Protection Agency and others to boost sustainability in all kinds of areas, from fashion (where even luxury brands like Balenciaga or Coach repurpose vintage clothing and bags) to architecture, where reusing wood, steel and bricks significantly reduces a building’s carbon footprint.
In addition to helping the planet, those who do it well can make a living from it. These days, Goosses and Widmann own a flourishing company: Airpaq. A crowdfunding campaign in 2017 yielded 70,000 Euros to get them started. Since then, they have upcycled 80,000 airbags, 100,000 seatbelts and 28,000 belt buckles – the equivalent of 60 tons of car trash.
For the successful upcycling, they received the 2021 German Design Award and, earlier this year, the renowned German Sustainability Award. The jurors evaluating the product commented that the startup “convinced us not only because of their uncompromising quality and functionality but also because of their ecological and ethical values….How well the startup translates upcycling and green fashion into an urban lifestyle brand is impressive.”
Despite their progress, Goosses and Widmann are up against a problem that’s immense: Cars are notoriously difficult to recycle because many parts are considered toxic waste. Therefore, up to 25% of vehicle scraps get shredded every year in Germany alone, the equivalent of over 501,000 tons. Because airbags and seatbelts are nearly indestructible, they are costly to recycle and almost always end up in landfills. Given that airbags and seatbelts save lives, they are subject to stringent security regulations, and manufacturers have a sky-high reject rate. “If a tiny filament protrudes somewhere, the manufacturer will throw out the entire output,” Widmann explains.
The nearly indestructible qualities that make this material very difficult to recycle render it an excellent resource for backpacks. “The material is so durable, you almost cannot tear it,” Goosses adds and demonstrates with a hard tug that even when the material already has a hole, it won’t rip it further. The material is also water repellent and extremely light.
The antique Singer is still in their Cologne headquarters but only as decoration. Their company with 12 employees is producing 500 backpacks and fanny packs every week in Romania, where the parts are professionally cut by laser, dyed and sewed. Airpaq still procures the belt buckles at scrapyards but they get most of the airbags directly from the reject pile of a nearby airbag producer. “We process the materials where they are produced,” Goosses explains. Only about 15 miles lie between one of Europe’s biggest airbag manufacturers and the Airpaq seamsters in Romania.

Co-founders Adrian Goosses and Michael Widmann demonstrate their company's equation: airbag plus seatbelt equals a backpack that's durable and eco-friendly.
Airpaq
The founders are aware that with price tags ranging from 100 to 160 Euro - a cost that reflects their intensive production process - Airpaq’s bags are hardly competitive. After all, anybody can buy a discount backpack for a fraction of the cost. So they recently added fanny packs for 30 Euro to their product line. Goosses and Widmann know they will need to lower their prices in the long run if they want to expand. Among other things, they didn’t pay themselves salaries during the first two years after founding the company.
Money-making isn’t their only objective. “Of course, it would be cheaper if we did what almost all textile producers do and move production to Asia,” Goosses says. That wasn’t an option for him. “Ship trash to Vietnam and let seamsters sew it together for cheap? No way, that would be anything but sustainable,” he says.
Michael Widmann’s family was already operating a textile production in Romania, mainly producing thin, elastic sports fashion. The family allowed Widmann and Goosses to produce their first professional prototypes there, but then the two youngsters had to buy their own machines, acquire the necessary knowhow, and hire their staff. They both moved to Romania for six months “to get to know the people behind the machines.” The founders emphasize that they pay fair wages, use eco-certified dyes and clean their own wastewater. “Normal production uses five to six liters of water per kilo material,” Widmann explains. “We only need a fraction because we massage the dye into the material by hand: 100 ml water for washing and dying per kilo.”
However, every time they return to the scrapyard, the abundance of trash sparks new ideas. “When you see how much material ends up there…” Widmann says, shaking his head without finishing the sentence. Goosses picks up the train of thought: “We want to make upcycling the new standard. You just have to be creative to get upcycling into the mainstream.”
And maybe they’ll return to their roots and finally find an idea for the tires after all. “One could turn the rubber into soles for comfortable shoes,” Widmann thinks out loud.
Recent advancements in engineering mean that the first preclinical trials for an artificial kidney could happen as soon as 18 months from now
Like all those whose kidneys have failed, Scott Burton’s life revolves around dialysis. For nearly two decades, Burton has been hooked up (or, since 2020, has hooked himself up at home) to a dialysis machine that performs the job his kidneys normally would. The process is arduous, time-consuming, and expensive. Except for a brief window before his body rejected a kidney transplant, Burton has depended on machines to take the place of his kidneys since he was 12-years-old. His whole life, the 39-year-old says, revolves around dialysis.
“Whenever I try to plan anything, I also have to plan my dialysis,” says Burton says, who works as a freelance videographer and editor. “It’s a full-time job in itself.”
Many of those on dialysis are in line for a kidney transplant that would allow them to trade thrice-weekly dialysis and strict dietary limits for a lifetime of immunosuppressants. Burton’s previous transplant means that his body will likely reject another donated kidney unless it matches perfectly—something he’s not counting on. It’s why he’s enthusiastic about the development of artificial kidneys, small wearable or implantable devices that would do the job of a healthy kidney while giving users like Burton more flexibility for traveling, working, and more.
Still, the devices aren’t ready for testing in humans—yet. But recent advancements in engineering mean that the first preclinical trials for an artificial kidney could happen as soon as 18 months from now, according to Jonathan Himmelfarb, a nephrologist at the University of Washington.
“It would liberate people with kidney failure,” Himmelfarb says.
An engineering marvel
Compared to the heart or the brain, the kidney doesn’t get as much respect from the medical profession, but its job is far more complex. “It does hundreds of different things,” says UCLA’s Ira Kurtz.
Kurtz would know. He’s worked as a nephrologist for 37 years, devoting his career to helping those with kidney disease. While his colleagues in cardiology and endocrinology have seen major advances in the development of artificial hearts and insulin pumps, little has changed for patients on hemodialysis. The machines remain bulky and require large volumes of a liquid called dialysate to remove toxins from a patient’s blood, along with gallons of purified water. A kidney transplant is the next best thing to someone’s own, functioning organ, but with over 600,000 Americans on dialysis and only about 100,000 kidney transplants each year, most of those in kidney failure are stuck on dialysis.
Part of the lack of progress in artificial kidney design is the sheer complexity of the kidney’s job. Each of the 45 different cell types in the kidney do something different.
Part of the lack of progress in artificial kidney design is the sheer complexity of the kidney’s job. To build an artificial heart, Kurtz says, you basically need to engineer a pump. An artificial pancreas needs to balance blood sugar levels with insulin secretion. While neither of these tasks is simple, they are fairly straightforward. The kidney, on the other hand, does more than get rid of waste products like urea and other toxins. Each of the 45 different cell types in the kidney do something different, helping to regulate electrolytes like sodium, potassium, and phosphorous; maintaining blood pressure and water balance; guiding the body’s hormonal and inflammatory responses; and aiding in the formation of red blood cells.

There's been little progress for patients during Ira Kurtz's 37 years as a nephrologist. Artificial kidneys would change that.
UCLA
Dialysis primarily filters waste, and does so well enough to keep someone alive, but it isn’t a true artificial kidney because it doesn’t perform the kidney’s other jobs, according to Kurtz, such as sensing levels of toxins, wastes, and electrolytes in the blood. Due to the size and water requirements of existing dialysis machines, the equipment isn’t portable. Physicians write a prescription for a certain duration of dialysis and assess how well it’s working with semi-regular blood tests. The process of dialysis itself, however, is conducted blind. Doctors can’t tell how much dialysis a patient needs based on kidney values at the time of treatment, says Meera Harhay, a nephrologist at Drexel University in Philadelphia.
But it’s the impact of dialysis on their day-to-day lives that creates the most problems for patients. Only one-quarter of those on dialysis are able to remain employed (compared to 85% of similar-aged adults), and many report a low quality of life. Having more flexibility in life would make a major different to her patients, Harhay says.
“Almost half their week is taken up by the burden of their treatment. It really eats away at their freedom and their ability to do things that add value to their life,” she says.
Art imitates life
The challenge for artificial kidney designers was how to compress the kidney’s natural functions into a portable, wearable, or implantable device that wouldn’t need constant access to gallons of purified and sterilized water. The other universal challenge they faced was ensuring that any part of the artificial kidney that would come in contact with blood was kept germ-free to prevent infection.
As part of last year’s KidneyX Prize, a partnership between the U.S. Department of Health and Human Services and the American Society of Nephrology, inventors were challenged to create prototypes for artificial kidneys. Himmelfarb’s team at the University of Washington’s Center for Dialysis Innovation won the prize by focusing on miniaturizing existing technologies to create a portable dialysis machine. The backpack sized AKTIV device (Ambulatory Kidney to Increase Vitality) will recycle dialysate in a closed loop system that removes urea from blood and uses light-based chemical reactions to convert the urea to nitrogen and carbon dioxide, which allows the dialysate to be recirculated.
Himmelfarb says that the AKTIV can be used when at home, work, or traveling, which will give users more flexibility and freedom. “If you had a 30-pound device that you could put in the overhead bins when traveling, you could go visit your grandkids,” he says.
Kurtz’s team at UCLA partnered with the U.S. Kidney Research Corporation and Arkansas University to develop a dialysate-free desktop device (about the size of a small printer) as the first phase of a progression that will he hopes will lead to something small and implantable. Part of the reason for the artificial kidney’s size, Kurtz says, is the number of functions his team are cramming into it. Not only will it filter urea from blood, but it will also use electricity to help regulate electrolyte levels in a process called electrodeionization. Kurtz emphasizes that these additional functions are what makes his design a true artificial kidney instead of just a small dialysis machine.
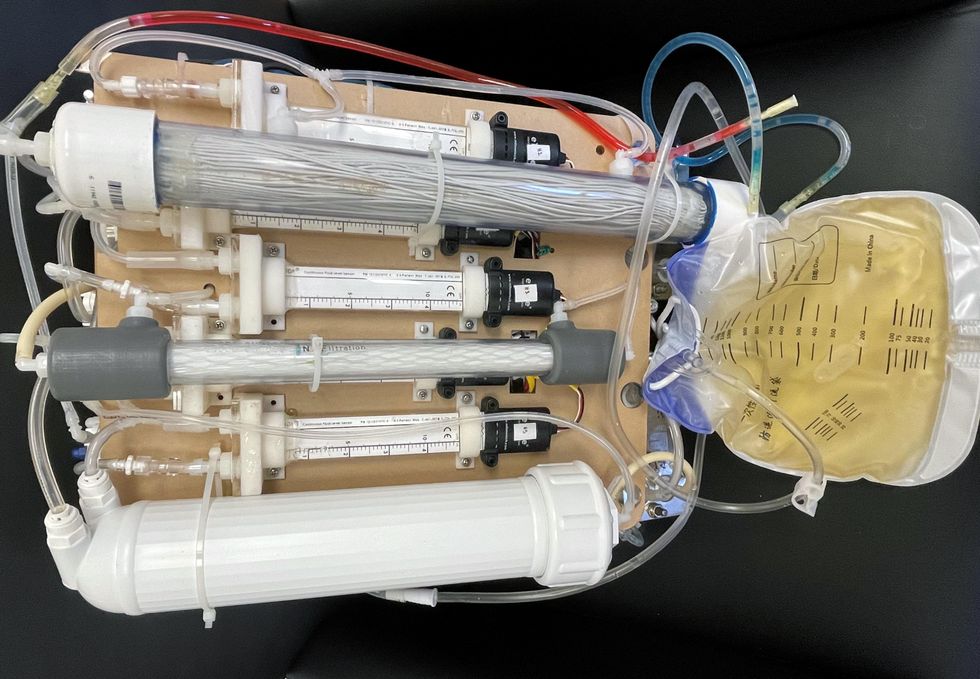
One version of an artificial kidney.
UCLA
“It doesn't have just a static function. It has a bank of sensors that measure chemicals in the blood and feeds that information back to the device,” Kurtz says.
Other startups are getting in on the game. Nephria Bio, a spinout from the South Korean-based EOFlow, is working to develop a wearable dialysis device, akin to an insulin pump, that uses miniature cartridges with nanomaterial filters to clean blood (Harhay is a scientific advisor to Nephria). Ian Welsford, Nephria’s co-founder and CTO, says that the device’s design means that it can also be used to treat acute kidney injuries in resource-limited settings. These potentials have garnered interest and investment in artificial kidneys from the U.S. Department of Defense.
For his part, Burton is most interested in an implantable device, as that would give him the most freedom. Even having a regular outpatient procedure to change batteries or filters would be a minor inconvenience to him.
“Being plugged into a machine, that’s not mimicking life,” he says.